A dissolving microneedle system incorporating dexamethasone-loaded nanoparticles has been developed to enhance therapeutic outcomes for inflammatory skin diseases by offering controlled drug delivery and improved patient compliance.
Advances in Transdermal Delivery
Inflammatory skin diseases, such as psoriasis and atopic dermatitis, affect millions of individuals worldwide and are typically managed with topical corticosteroids due to their anti-inflammatory and immunosuppressive properties. However, these treatments are limited by inadequate skin penetration, systemic side effects, and poor patient compliance stemming from the greasy texture and frequent application required.
To address these challenges, researchers have turned to dissolving microneedles, a minimally invasive transdermal drug delivery system. These micron-sized devices penetrate the skin’s stratum corneum without causing significant pain or damage, dissolving upon contact with skin fluids to release their drug payload directly into the dermis. This approach offers advantages such as controlled drug release, enhanced bioavailability, and improved patient adherence.
A New Approach to Enhanced Drug Delivery
This study aimed to develop and evaluate a novel system combining dissolving microneedles made of sodium alginate with dexamethasone-loaded PLGA (poly(lactic-co-glycolic acid)) nanoparticles. Researchers Hala Dawud and Aiman Abu Ammar from the Azrieli College of Engineering in Jerusalem designed this system to address the limitations of current corticosteroid therapies. The work was published in Pharmaceutics in February 2023, detailing a minimally invasive strategy for improving the delivery and efficacy of topical corticosteroids.

The system was designed to achieve controlled drug release, increase skin penetration efficiency, and enhance patient convenience by allowing self-administration. By embedding nanoparticles into the microneedle tips, the system reduces drug wastage and ensures site-specific delivery, bypassing the limitations of conventional creams and ointments.
Research Methods & Results
Experimental Process Outline
- Preparation of Dexamethasone-Loaded Nanoparticles (DEX-NPs): DEX was encapsulated into PLGA nanoparticles using a nanoprecipitation method, achieving high encapsulation efficiency.
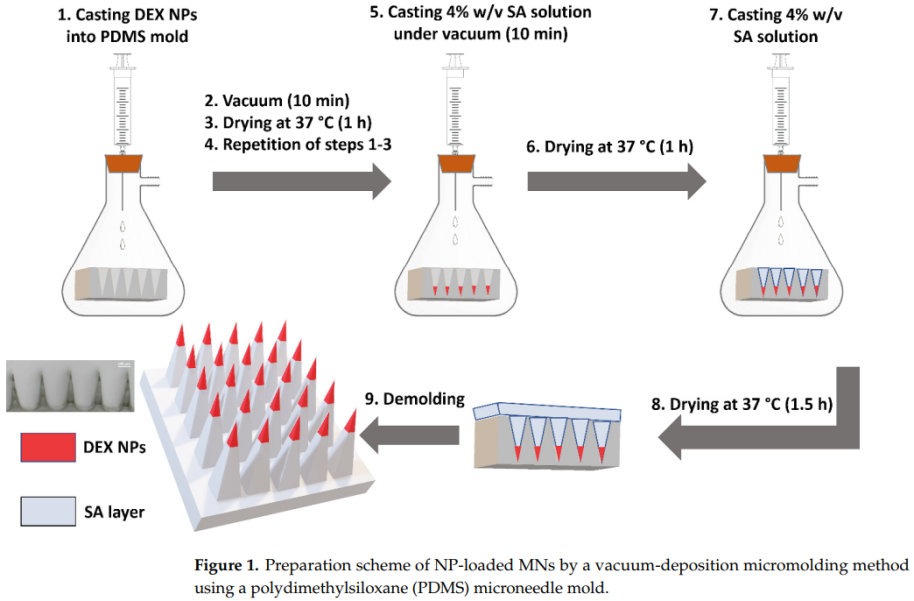
- Fabrication of Microneedles (MNs): Sodium alginate was used to construct microneedles via vacuum-deposition micromolding, embedding the nanoparticles into the needle tips.
- Characterization: The mechanical strength, dissolution rate, and insertion efficiency of the microneedles were evaluated using various models.
- In Vitro Drug Release Studies: The drug release profile was assessed using a dialysis bag method.
Key Experiment Details and Results
1. Preparation of DEX-Loaded Nanoparticles (DEX-NPs)
Dexamethasone was encapsulated in PLGA using a nanoprecipitation method. The resulting nanoparticles exhibited an average diameter of 93.7 ± 5.1 nm, a polydispersity index of 0.27 ± 0.04, and a zeta potential of -27.5 ± 3.31 mV. The encapsulation efficiency was 80 ± 0.6%, with a drug loading content of 9.1 ± 0.1%. These values confirm the system’s suitability for controlled drug delivery by ensuring effective drug encapsulation and sustained release.
2. Fabrication of Microneedles (MNs)
Microneedles were fabricated using sodium alginate, incorporating the nanoparticles into the needle tips via a vacuum-deposition micromolding method. The needles were approximately 500 µm tall with a base width of 200 µm. The addition of nanoparticles improved the mechanical strength and insertion efficiency compared to sodium alginate-only microneedles. The MNs dissolved within 15 seconds when applied to ex vivo chicken skin.
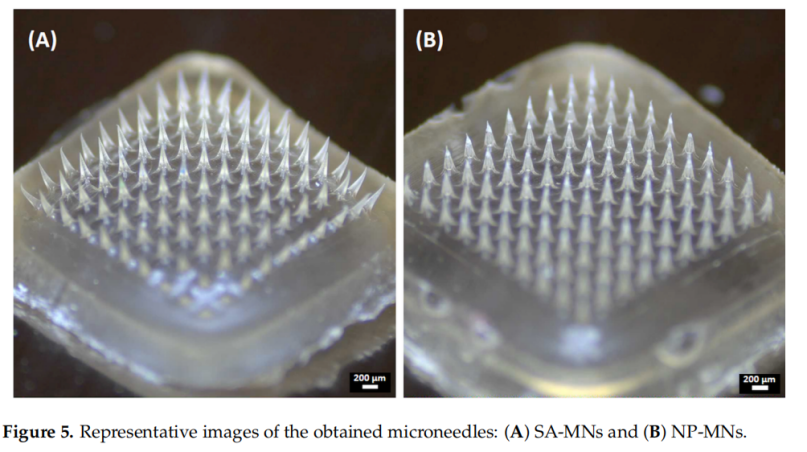
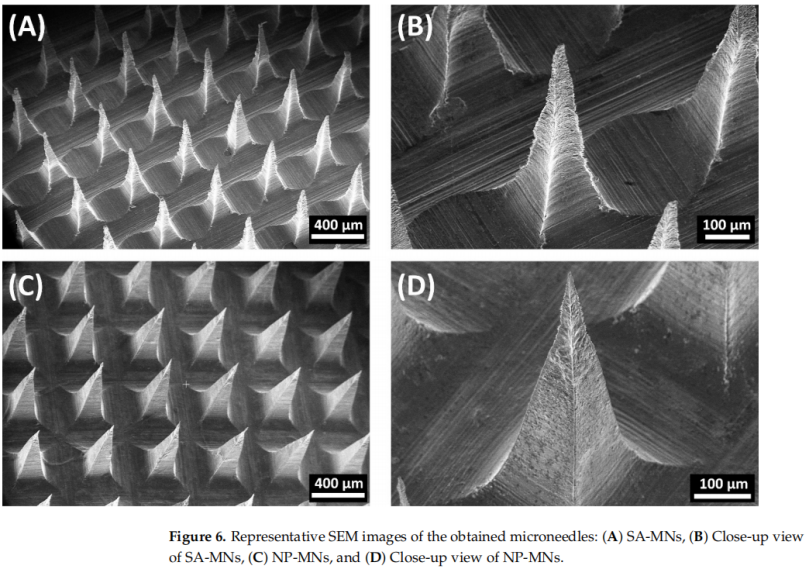
3. Mechanical and Insertion Properties
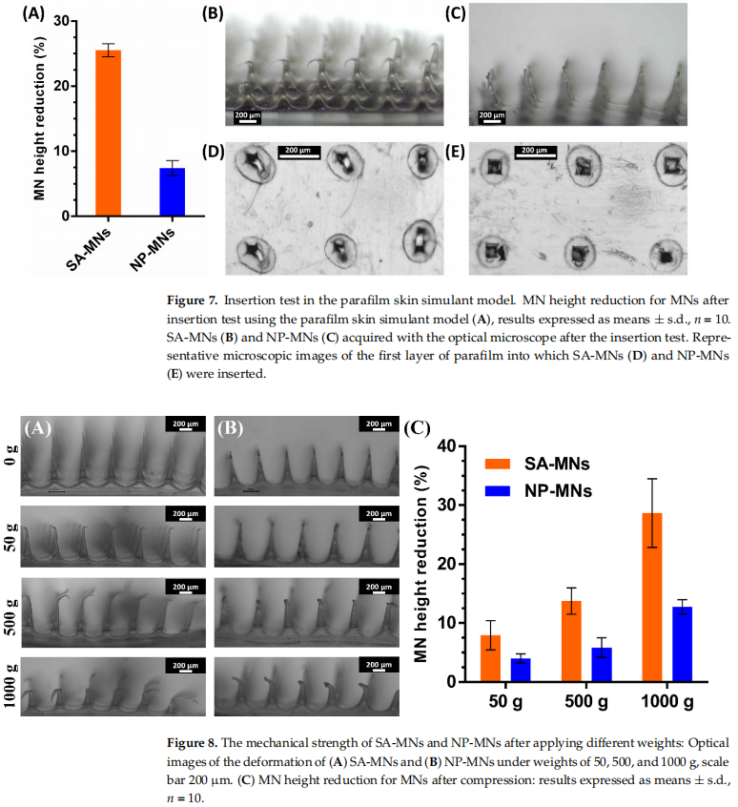
The microneedles demonstrated adequate mechanical strength to withstand static forces up to 1000 g without fracturing. When inserted into parafilm layers simulating skin, the NP-MNs created uniform pores with minimal height reduction (7.4%), highlighting their structural integrity and effective skin penetration capabilities.
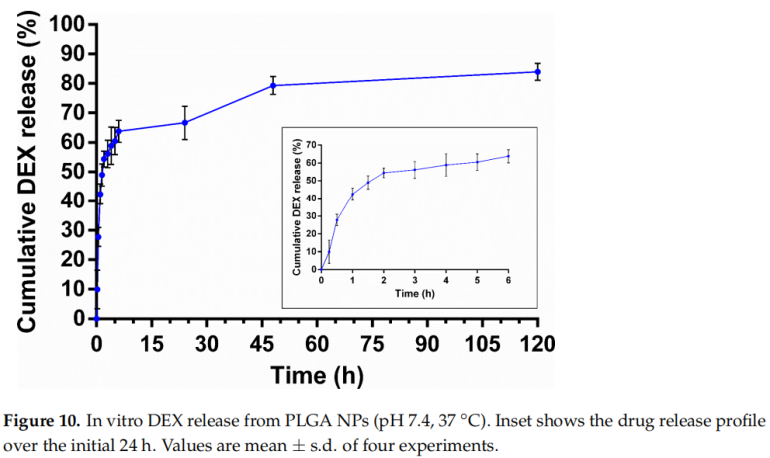
4. In Vitro Drug Release Studies
The drug release from nanoparticles followed a biphasic pattern, with an initial burst release in the first 6 hours followed by sustained release over 120 hours. This release profile is advantageous for addressing both acute and chronic inflammation associated with skin diseases.
Key Technical Points and Innovations
- Integration of dexamethasone-loaded PLGA nanoparticles into dissolving microneedles.
- Improved mechanical strength and rapid dissolution of microneedles.
- Controlled drug release over 120 hours, enhancing therapeutic efficiency and patient compliance.
Findings and Future Directions
This study successfully demonstrated the feasibility of using dissolving microneedles loaded with dexamethasone-PLGA nanoparticles for the treatment of inflammatory skin diseases. The microneedles achieved controlled drug delivery, rapid dissolution, and efficient skin penetration, addressing limitations of conventional corticosteroid therapies.
The authors noted that the proposed system could improve patient adherence and reduce side effects associated with systemic drug exposure. However, further in vivo studies are required to confirm its efficacy and safety in clinical settings. Additionally, future research should explore its application for other drugs and therapeutic areas.
This innovative approach presents a promising minimally invasive alternative for managing inflammatory skin diseases, paving the way for more effective and patient-centered therapies.
Reference:
Dawud, Hala, and Aiman Abu Ammar. “Rapidly dissolving microneedles for the delivery of steroid-loaded nanoparticles intended for the treatment of inflammatory skin diseases.” Pharmaceutics 15.2 (2023): 526.
