A Historical Perspective on Immunotherapy
Immunotherapy has revolutionized cancer treatment by harnessing the body’s immune system to target malignancies. For prostate cancer patients, especially those with bone metastases, immunotherapy offers renewed hope. However, its efficacy has been limited in treating bone metastatic castration-resistant prostate cancer (mCRPC) due to the complex and immunosuppressive tumor microenvironment.
Current Innovations in Nanomedicine
Recent advancements in nanotechnology have paved the way for more precise and targeted cancer therapies. Researchers are now leveraging nanomedicine to overcome the challenges of drug delivery and immune resistance, opening a new frontier in cancer treatment.
The Persistent Challenge of Bone Metastatic Prostate Cancer
Bone metastases affect up to 90% of mCRPC patients, significantly reducing survival rates and quality of life. Existing treatments are insufficient, necessitating innovative solutions to manage the immunosuppressive bone microenvironment and improve therapeutic outcomes.
A New Era in Cancer Treatment: The MTO@PA/Fe3+ MOF Nanosystem
Pioneering Research by Shu Huang and Team
Published in Cancer Nanotechnology in 2023, the research led by Shu Huang from Hunan Provincial People’s Hospital and Central South University introduces a groundbreaking nano-regulator system, MTO@PA/Fe3+ MOF. This nanomedicine combines advanced drug delivery with immune regulation, offering a dual-action approach to tackle mCRPC.
Addressing Critical Needs
The MTO@PA/Fe3+ MOF system is designed to overcome the limitations of conventional therapies. It not only improves drug delivery to the bone metastatic site but also restores immune sensitivity by blocking the immunosuppressive TGF-β pathway.
Breaking Down the Science: Methodology
Innovative Design of the Nano-Regulator
The nanosystem is crafted by assembling phytic acid (PA) and iron (Fe3+) into a metal-organic framework (MOF), which encapsulates the chemotherapy drug mitoxantrone (MTO). This design ensures high drug-loading capacity, bone-targeting efficiency, and prolonged systemic circulation.
Comprehensive Evaluation
The study employed in vitro and in vivo techniques to assess the nanosystem’s efficacy. Key experiments included tumor cell cytotoxicity assays, animal models for bone metastatic prostate cancer, and biodistribution studies to measure drug targeting and therapeutic impact.
Results: Groundbreaking Discoveries in Nanomedicine
Key Findings: Tumor Targeting and Immune Modulation
The MTO@PA/Fe3+ MOF nanosystem showcased a dual action in combating bone metastatic prostate cancer. It demonstrated selective cytotoxicity against tumor cells while sparing immune cells, significantly improving the therapeutic index.
Selective Cytotoxicity: The nanosystem achieved higher cytotoxicity in prostate cancer cells (RM-1) compared to free mitoxantrone (MTO). The IC50 values were 23.2 nM for the nanosystem and 34.9 nM for free MTO, illustrating enhanced delivery and efficacy.
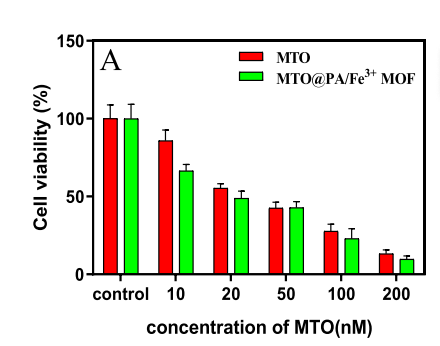
Immune Sensitization: By inducing the ubiquitination and degradation of TGF-β receptors, the nanosystem suppressed the immunosuppressive TGF-β pathway. This enhanced the efficacy of immune checkpoint inhibitors like αCTLA-4, a pivotal advancement for immunotherapy.
Tumor Immunogenicity: The nanosystem triggered immunogenic cell death (ICD) in tumor cells, evidenced by increased calreticulin (CRT) exposure, HMGB1 expression, and ATP release. These markers stimulate immune responses, further boosting anti-tumor immunity.
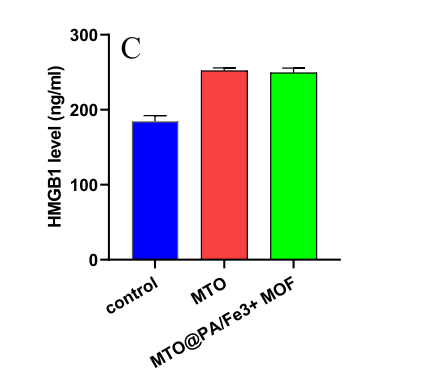
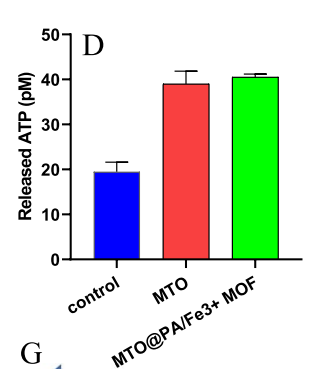
Quantitative Highlights
- Enhanced Drug Accumulation: Drug concentration in the bone metastatic site was threefold higher for the nanosystem than free MTO, confirming superior targeting efficiency (Figure 3D).
- Tumor Volume Suppression: In vivo, tumor growth was significantly inhibited in mice treated with the MTO@PA/Fe3+ MOF and αCTLA-4 combination therapy. Tumor volume was reduced compared to controls.
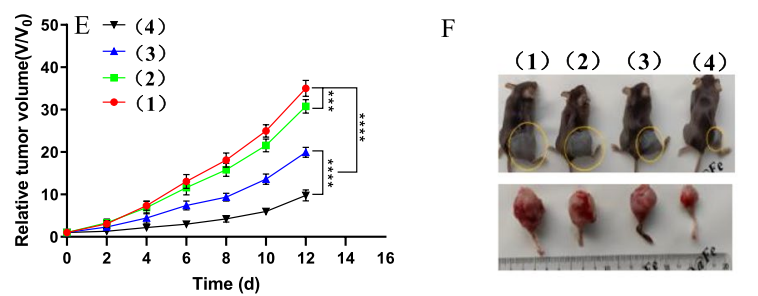
- Bone Preservation: The combination therapy also mitigated skeletal-related events such as bone destruction, as observed through micro-CT imaging .
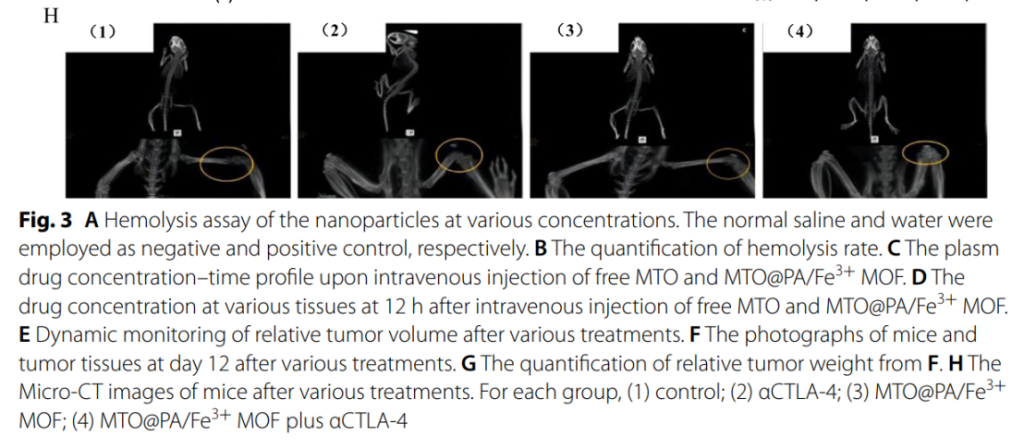
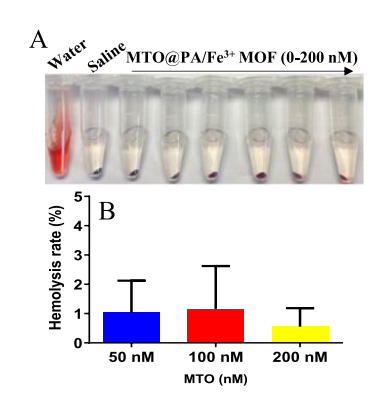
Safety and Biocompatibility
The nanosystem exhibited minimal toxicity in mice. Hemolysis rates were below 5% across tested concentrations,
and biochemical parameters like ALT, AST, BUN, and CRE remained within normal ranges after treatment

Data Highlights
The study reported a threefold increase in drug accumulation at the bone metastatic site and substantial tumor suppression when combined with αCTLA-4 therapy. These results underscore the potential of MTO@PA/Fe3+ MOF to redefine immunotherapy for metastatic cancer.
Looking Ahead: Implications and Future Directions
Transforming Prostate Cancer Treatment
This research represents a significant leap forward in using nanotechnology to enhance immunotherapy. The MTO@PA/Fe3+ MOF nanosystem holds promise for clinical translation, offering a lifeline for patients with limited treatment options.
Addressing Limitations
While the findings are promising, the study acknowledges limitations such as the need for long-term safety data and broader clinical trials to validate efficacy in diverse patient populations.
Pathways for Future Research
Future studies could explore the integration of other therapeutic agents and the application of this nanosystem to other metastatic cancers, further expanding its clinical potential.
Conclusion: A Beacon of Hope in Cancer Care
The development of the MTO@PA/Fe3+ MOF nanosystem heralds a new era in cancer treatment. By combining targeted drug delivery with immune regulation, this innovation not only enhances therapeutic efficacy but also offers a blueprint for addressing complex oncological challenges.
Reference:
Huang, Shu, et al. “Targeting nano-regulator based on metal–organic frameworks for enhanced immunotherapy of bone metastatic prostate cancer.” Cancer Nanotechnology 14.1 (2023): 43.
