According to a phase I clinical trial published in Nature, a novel personalized RNA neoantigen vaccine combined with immunotherapy and chemotherapy has demonstrated significant potential to delay pancreatic cancer.
Background
Pancreatic ductal adenocarcinoma (PDAC) ranks as the third leading cause of cancer-related mortality in the United States and seventh globally. This aggressive malignancy is marked by an alarmingly low survival rate of 12%, which has remained largely unchanged over six decades. Surgery is currently the only curative treatment option; however, even after successful resection, up to 90% of patients experience disease recurrence within 7 to 9 months. Conventional treatments, including multi-agent chemotherapy and radiation therapy, provide limited long-term benefits, with immune checkpoint inhibitors demonstrating minimal efficacy in PDAC due to its low mutation burden and immunologically “cold” tumor microenvironment.
Research Aim and Objectives
The study sought to evaluate the potential of autogene cevumeran, an individualized mRNA neoantigen vaccine, to stimulate neoantigen-specific T-cell responses and delay recurrence in PDAC patients. The vaccine was developed to encode up to 20 patient-specific tumor neoantigens, aiming to overcome the immune evasion typical of this cancer type. The trial integrated the vaccine into a treatment regimen with atezolizumab (anti-PD-L1 immunotherapy) and mFOLFIRINOX (a chemotherapy protocol) to amplify immune responses and assess feasibility, safety, and immunogenicity. The study was conducted by researchers from Memorial Sloan Kettering Cancer Center in collaboration with BioNTech and Genentech, with findings published in the June 1, 2023, issue of Nature.

Research Methods and Experimental Design
The phase I trial enrolled 16 patients with surgically resectable PDAC, all meeting criteria such as having at least five neoantigens and no prior neoadjuvant therapy. After tumor resection, patients underwent sequential treatment:
- Atezolizumab: One dose at week 6 to modulate immune checkpoint activity.
- Autogene Cevumeran: A personalized vaccine delivered as eight priming doses (weeks 9 to 17) and a booster dose at week 46.
- mFOLFIRINOX: Chemotherapy commenced at week 21, comprising 12 biweekly cycles.
Key experimental techniques included:
- Neoantigen Selection and Vaccine Synthesis: Tumor and normal DNA/RNA were sequenced to identify mutations. Neoantigens were ranked by predicted immunogenicity and encoded into uridine mRNA–lipoplex nanoparticles for rapid synthesis.
- CloneTrack: This innovative method utilized T-cell receptor sequencing and computational modeling to track vaccine-expanded T-cell clones, quantifying their expansion in blood.
- ELISpot Assay: An established method to detect high-magnitude interferon-γ (IFNγ) secretion as a marker of neoantigen-specific T-cell activation.
This rigorous experimental framework enabled real-time vaccine production and integration into clinical workflows, a significant advancement in personalized immunotherapy.
Results and Implications
The trial demonstrated promising results in terms of immune response and clinical feasibility:
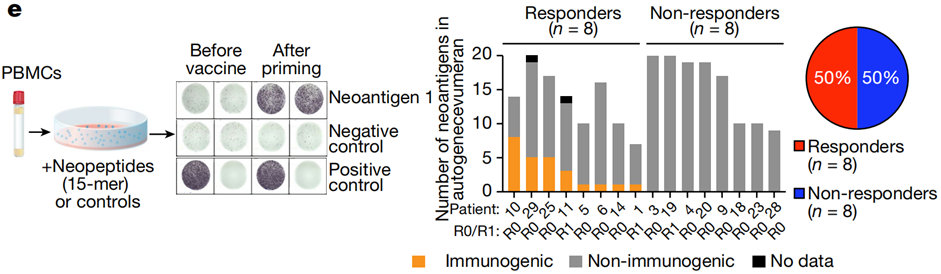
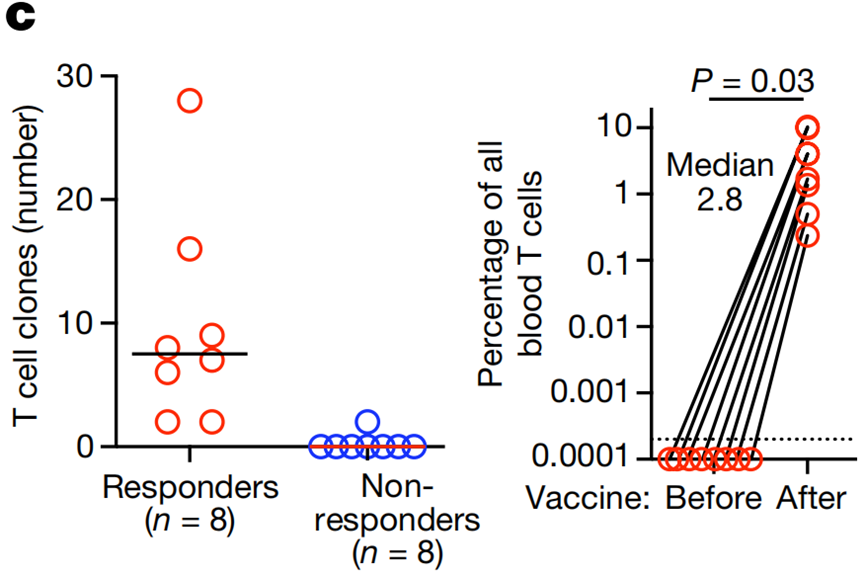
- Immune Responses: Eight out of 16 patients (50%) showed vaccine-induced neoantigen-specific T-cell activity. These responders exhibited de novo expansion of neoantigen-specific T-cells, which constituted up to 10% of circulating T-cells in some cases.
- Recurrence-Free Survival (RFS): Responders achieved a median RFS not reached at the 18-month follow-up, compared to 13.4 months in non-responders (P = 0.003, HR = 0.08; 95% CI 0.01–0.4). Landmark analysis corroborated these findings.

- Safety: The vaccine was well-tolerated, with one patient experiencing grade 3 adverse events (fever and hypertension). Most patients reported only mild to moderate side effects.
Key data included:
- T-Cell Expansion: Median 2.8% of total blood T-cells in responders, with a peak expansion of 10%.
- Neoantigen-Specific Responses: 25 out of 230 administered neoantigens (11%) elicited measurable immune responses.
- Adverse Events: No grade 3 or higher adverse events were associated with atezolizumab.
Summary
The authors concluded that autogene cevumeran, as part of a combined treatment regimen, was both safe and feasible, effectively inducing robust neoantigen-specific T-cell responses in resectable PDAC patients. These responses were associated with prolonged recurrence-free survival, underscoring the vaccine’s potential in addressing a critical gap in PDAC treatment.
Limitations: The study’s small sample size and demographic homogeneity (primarily White participants) limit generalizability. Additionally, the marginal enrichment of splenectomized patients among non-responders raises questions about patient selection.
Future Directions: The authors emphasized the need for larger, more diverse trials and further optimization of vaccine design and production timelines. They suggested exploring biomarkers to identify patients most likely to benefit and expanding neoantigen discovery to include structural variants like fusions. Broader application of this approach to other low-immunogenicity cancers is also warranted.
Reference:
Rojas, Luis A., et al. “Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer.” Nature 618.7963 (2023): 144-150.
