Introduction: A New Era in Tumor Targeting
For decades, the field of nanomedicine has aspired to revolutionize cancer therapy with selective tumor-targeting nanoparticles. Despite significant research, a persistent challenge has been the inability to achieve precise targeting in vivo. The problem lies in the non-selectivity of nanoparticles, often attributed to insufficient characterization of surface ligands. Prostate cancer, the second most common cause of cancer-related deaths in men, underscores this urgency. While conventional treatments like surgery and chemotherapy have provided some relief, they lack targeted precision, leading to severe side effects and limited success in advanced cases.
Recent advancements in prostate cancer-targeting nanoparticles present a promising avenue. By harnessing multivalent interactions—where multiple ligands on a nanoparticle bind simultaneously to receptors—scientists aim to improve cellular uptake and targeting precision. However, a major bottleneck remains: accurately functionalizing ligands on nanoparticle surfaces to ensure optimal targeting efficacy.
Behind the Breakthrough: The Researchers Driving Innovation
The latest research, conducted by Madhura Murar, Silvia Pujals, and Lorenzo Albertazzi, represents a leap forward. Published in 2023 in Nanoscale Advances, their study builds on years of work in Spain and the Netherlands. The team is affiliated with leading institutes like the Institute for Bioengineering of Catalonia and the Eindhoven University of Technology. Their research centers on addressing a critical question: can multivalent nanoparticles functionalized with a WQP peptide effectively target prostate cancer by overcoming the limitations of conventional delivery methods?
Their work holds immense significance, offering a strategy to selectively target prostate-specific membrane antigen (PSMA), a biomarker overexpressed in most prostate cancer cells but minimally present in healthy tissues.
Innovative Methods: Building Precision-Engineered Nanoparticles
The team adopted a meticulous approach to develop multivalent nanoparticles. Their design incorporated WQP peptides at varying densities—5% and 30%—on polymeric nanoparticle surfaces to assess the effects of ligand density on targeting efficacy.
To quantify the number of WQP peptides on nanoparticle surfaces, they employed an innovative enzymatic digestion technique. This method, using chymotrypsin protease, allowed precise measurement of peptide density through advanced chromatography and mass spectrometry.
The researchers tested the nanoparticles’ targeting capabilities using prostate cancer and healthy cell lines. By analyzing cellular uptake across cells with varying PSMA expression, they validated the selective targeting potential of their nanoparticle formulations.
Results: Multivalency Drives Enhanced Uptake and Selectivity
The study’s results demonstrated the significant advantages of multivalent nanoparticles over their monovalent counterparts in prostate cancer targeting. The researchers synthesized nanoparticles with WQP peptide densities of 5% and 30%, assessing their performance across four cell lines: two prostate cancer cell lines with high (LNCaP) and moderate (22Rv1) PSMA expression, one prostate cancer cell line with low PSMA expression (PC3), and one healthy prostate cell line (RWPE1).
Enhanced Cellular Uptake with Higher WQP Valency
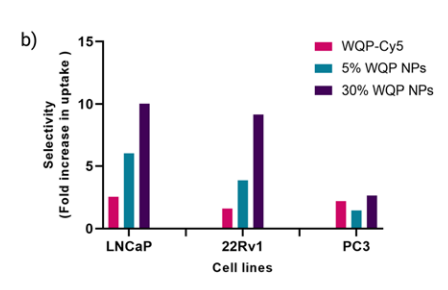
Quantitative analysis revealed that nanoparticles with higher WQP valency (30%) exhibited a substantial increase in cellular uptake compared to those with 5% valency. Specifically:
- In LNCaP cells, nanoparticles with 30% WQP valency achieved a 10-fold increase in uptake compared to the WQP monomer.
- In 22Rv1 cells, the uptake was approximately 9-fold higher for 30% valency nanoparticles relative to the monomer.
- A more moderate enhancement was observed in PC3 cells due to their lower PSMA expression, with uptake increasing 4-fold for 30% valency nanoparticles.
Flow cytometry data confirmed these findings, showing statistically significant differences in fluorescence intensity corresponding to the uptake of fluorescently tagged nanoparticles.
Impact of Surface Ligand Density
The researchers employed enzymatic digestion techniques to quantify the number of WQP peptides on nanoparticle surfaces. Interestingly, while nanoparticles with 30% valency had a higher overall number of WQP peptides, their surface coverage was less efficient than 5% valency nanoparticles. This discrepancy was attributed to steric hindrance at higher ligand densities, causing some peptides to embed within the nanoparticle core rather than remaining accessible on the surface.
This finding underscores the importance of optimizing ligand density to balance nanoparticle stability, accessibility of targeting ligands, and biological performance.
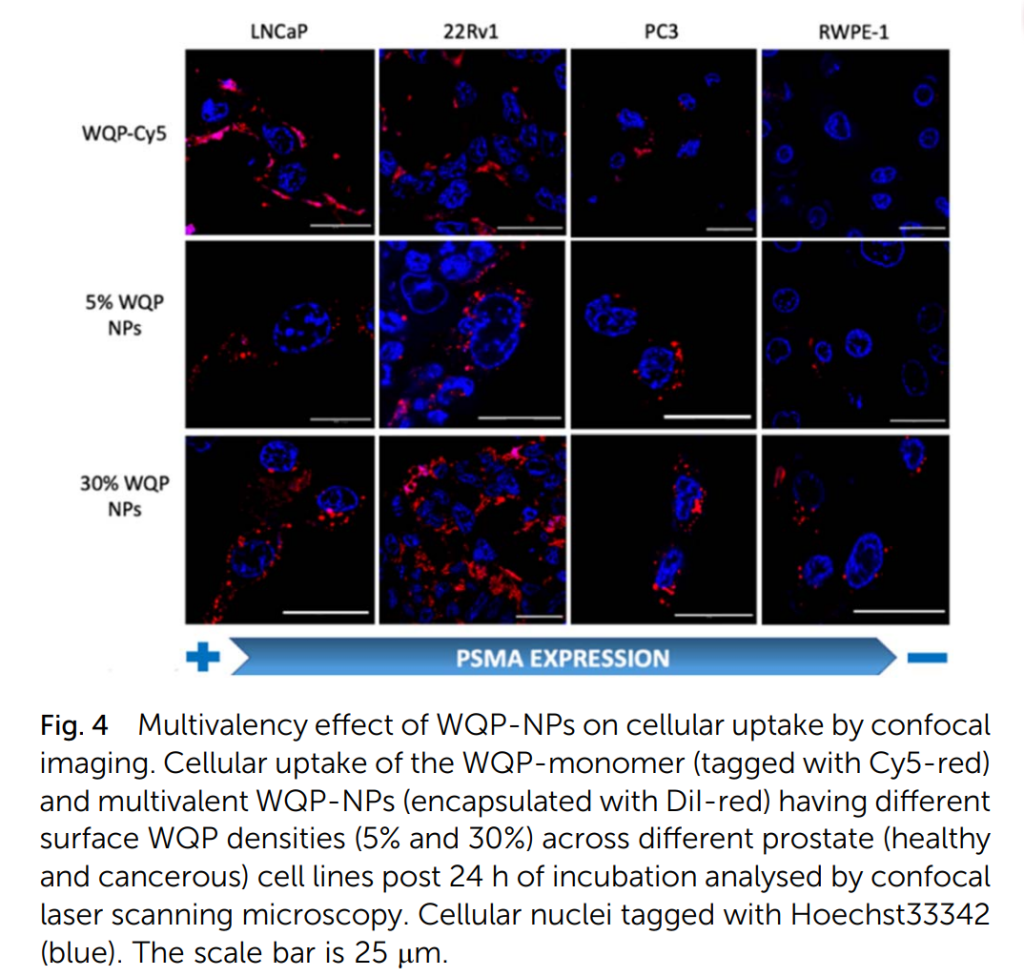
Microscopy and Imaging Insights
Confocal laser scanning microscopy provided a qualitative assessment of uptake patterns. The images revealed distinct red fluorescence from WQP-tagged nanoparticles in LNCaP and 22Rv1 cells, indicating strong binding and internalization. In contrast, the fluorescence was markedly weaker in PC3 and RWPE1 cells, aligning with their lower PSMA expression levels.
Overall, the results highlight the potential of multivalent nanoparticles to achieve highly selective and efficient targeting of prostate cancer cells, with 30% valency formulations showing the greatest promise for therapeutic applications. These findings pave the way for further exploration into ligand density optimization and multi-ligand strategies to enhance targeting specificity and therapeutic efficacy.
Paving the Path to Personalized Medicine
This groundbreaking research highlights the promise of multivalent nanoparticles in achieving selective and efficient prostate cancer targeting. By enhancing targeting precision and minimizing off-target effects, the approach paves the way for more effective, patient-specific treatments.
While the findings are encouraging, the study acknowledges challenges such as optimizing ligand density to balance targeting efficacy and nanoparticle stability. Future research should explore multi-ligand systems to further refine targeting specificity and broaden the therapeutic potential of these nanoparticles.
In conclusion, the work of Murar, Pujals, and Albertazzi is a testament to the transformative possibilities of nanomedicine. Their study lays the foundation for a future where cancer treatments are not only more effective but also tailored to individual patients, heralding a new era in personalized medicine.
Reference:
Murar, Madhura, Silvia Pujals, and Lorenzo Albertazzi. “Multivalent effect of peptide functionalized polymeric nanoparticles towards selective prostate cancer targeting.” Nanoscale advances 5.5 (2023): 1378-1385.
