A Legacy of Healing and New Challenges
Curcumin, derived from the rhizome of turmeric (Curcuma longa), has long been celebrated for its potent anti-inflammatory, antioxidant, and anti-rheumatic properties. Despite its vast therapeutic potential, curcumin’s clinical application has been limited by challenges such as low solubility, poor bioavailability, and rapid degradation in the body. Addressing these hurdles is crucial to unlocking its full potential in treating inflammatory and arthritic conditions that affect millions worldwide.
Innovative Research for a Brighter Future

In a groundbreaking study published in Scientific Reports, an international team of researchers led by Hafiz Muhammad Asif and Farah Zafar from The Islamia University of Bahawalpur, Pakistan, collaborated with Khalil Ahmad from the University of Management and Technology and Amjad Iqbal from institutions in Poland and Portugal. Their work focuses on enhancing curcumin’s therapeutic efficacy by encapsulating it within chitosan nanoparticles, a novel drug delivery system designed to overcome curcumin’s inherent limitations.
The study aimed to improve the solubility, stability, and bioavailability of curcumin using chitosan nanoparticles synthesized via the Ionic gelation method. This innovative approach could pave the way for more effective treatments for inflammatory disorders, including arthritis.
A Detailed and Rigorous Methodology
The research utilized the Ionic gelation method to create curcumin-loaded chitosan nanoparticles. By varying concentrations of chitosan and sodium tripolyphosphate (STPP) under controlled conditions, the team optimized four nanoparticle formulations. Characterization of the nanoparticles was conducted using Scanning Electron Microscopy (SEM) for morphology, Fourier Transform Infrared Spectroscopy (FTIR) for compatibility, and dynamic light scattering for size and zeta potential analysis.
To evaluate the nanoparticles’ effectiveness, in vitro tests assessed their anti-inflammatory and anti-arthritic properties. The human red blood cell (HRBC) membrane stabilization method and protein denaturation assays revealed significant improvements in therapeutic activity compared to pure curcumin.
Remarkable Results and Quantitative Insights
The study revealed several significant findings that underscore the effectiveness of curcumin-loaded chitosan nanoparticles. By improving the solubility, stability, and bioavailability of curcumin, the research demonstrated clear advantages over pure curcumin formulations. Key results include:
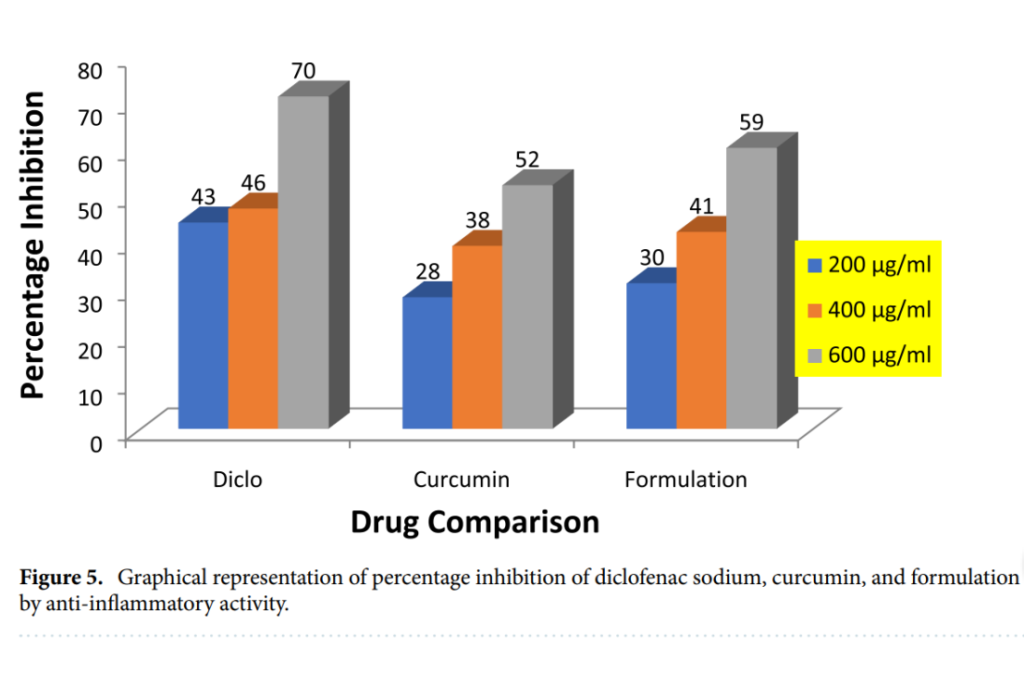
Enhanced Anti-inflammatory Activity: The HRBC membrane stabilization method showed that the nanoparticles achieved 59% inhibition at a concentration of 600 µg/ml. In comparison, pure curcumin displayed 52% inhibition, while the standard drug diclofenac sodium reached 70%. These results highlight the superior performance of the nanoparticle formulation. At lower concentrations of 200 µg/ml and 400 µg/ml, the nanoparticles exhibited 30% and 41% inhibition, compared to 28% and 38% for pure curcumin.
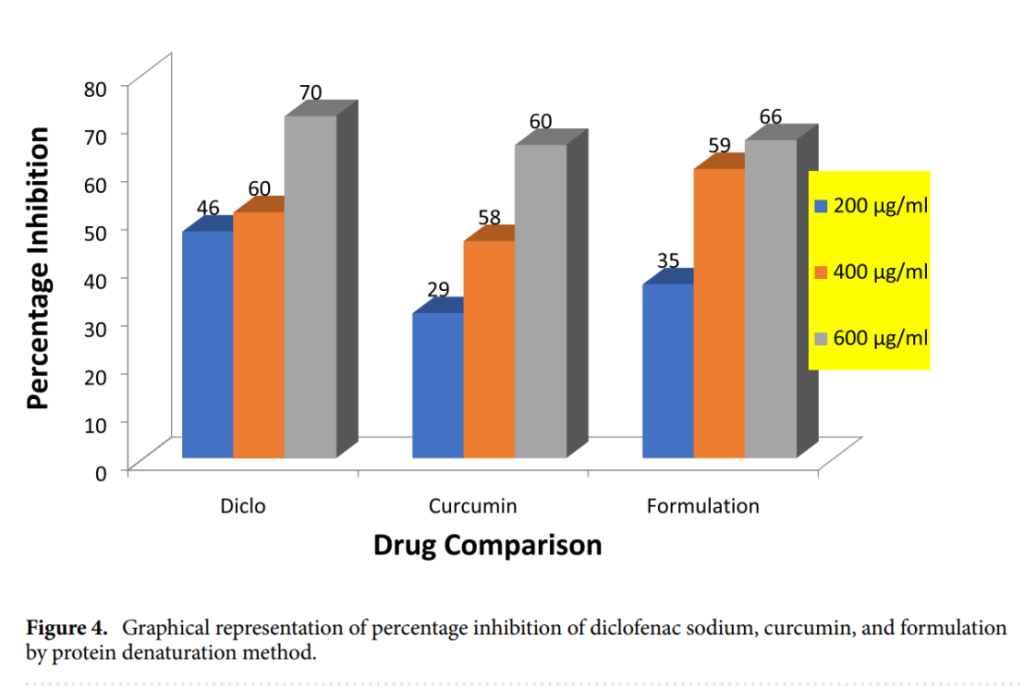
Superior Anti-arthritic Properties: Using the protein denaturation method, nanoparticles achieved a 66% inhibition rate at 600 µg/ml, outperforming curcumin at 60% and closely approaching the standard drug’s 70%. At lower concentrations of 200 µg/ml and 400 µg/ml, the nanoparticle formulation showed 35% and 59% inhibition, respectively, surpassing pure curcumin’s performance of 29% and 58%.
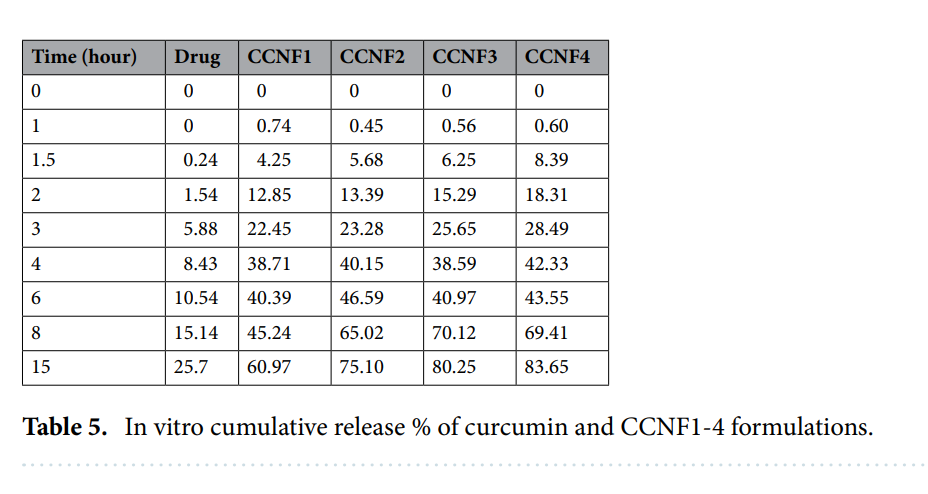
Improved Drug Release Profiles: In vitro drug release studies revealed a sustained and controlled release behavior. After 15 hours, the nanoparticles demonstrated a cumulative release of 60% to 83%, significantly higher than the 25% release from pure curcumin. Among the formulations, CCNF4 achieved the highest release (83%), followed by CCNF3 (80%), CCNF2 (75%), and CCNF1 (60%).
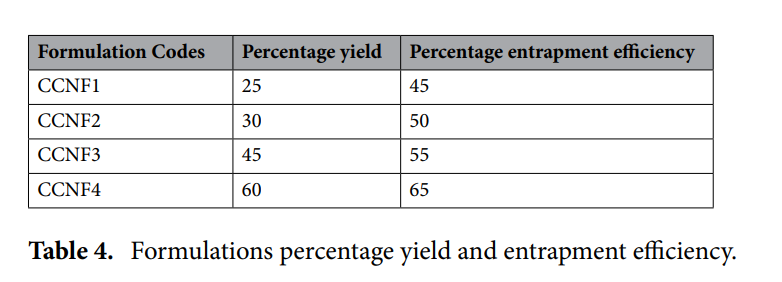
Optimized Formulation Characteristics: The nanoparticles exhibited an average size range of 481.7 nm to 761.5 nm across different formulations, with the smallest size and best dispersity index observed in CCNF1. Zeta potential measurements confirmed the stability of the formulations, with CCNF1 displaying a positive zeta potential of 37.7 mV. Entrapment efficiency ranged from 45% (CCNF1) to 65% (CCNF4), correlating with the nanoparticle yield, which varied from 25% to 60%.
These quantitative outcomes underline the success of the nanoparticle-based delivery system in enhancing the therapeutic efficacy of curcumin. The research also demonstrated the reproducibility and scalability of the Ionic gelation method for developing advanced drug delivery systems.
Paving the Way for Future Innovations
This study marks a significant advancement in the application of nanotechnology to address long-standing challenges in drug delivery. By encapsulating curcumin within biocompatible chitosan nanoparticles, the research offers a promising strategy to enhance treatment outcomes for inflammatory and arthritic conditions.
While the findings are promising, the authors acknowledge limitations such as the need for in vivo studies and clinical trials to validate the nanoparticles’ efficacy and safety. Future research could explore further optimization of the nanoparticle formulations and their application to other therapeutic areas.
The study represents a pivotal step toward harnessing curcumin’s full therapeutic potential, offering hope for millions affected by chronic inflammatory diseases. With further development, this innovative approach could revolutionize drug delivery and therapeutic strategies in modern medicine.
Reference:
Asif, Hafiz Muhammad, et al. “Synthesis, characterization and evaluation of anti-arthritic and anti-inflammatory potential of curcumin loaded chitosan nanoparticles.” Scientific Reports 13.1 (2023): 10274.
