Introduction: Tackling the Chronic Kidney Disease Conundrum
Renal fibrosis, a hallmark of chronic kidney disease (CKD), has long posed a challenge to medical science. Traditional therapies have focused on slowing disease progression rather than offering a definitive cure. With an estimated 15% of the global population affected by CKD, the urgency for innovative treatments has never been greater. Recent advancements in nanotechnology have paved the way for the use of engineered extracellular vesicles (EVs) as therapeutic carriers, promising targeted and efficient solutions. However, the difficulty of ensuring these therapies reach and remain effective at the site of kidney injury continues to hinder progress.
Research Objectives: Innovating with SPION-Enhanced MSC-EVs

A research team led by Cheng Ji and colleagues from Jiangsu University has developed a cutting-edge solution to this challenge. Their study, published in npj Regenerative Medicine, explores the use of superparamagnetic iron oxide nanoparticles (SPION) to enhance mesenchymal stem cell-derived extracellular vesicles (MSC-EVs). By overexpressing the carboxyl terminus of Hsc70-interacting protein (CHIP) within these vesicles, the team aims to deliver potent anti-fibrotic effects directly to damaged renal tissue. This novel approach combines biological ingenuity with nanotechnology to create a targeted and efficient therapeutic platform.
Methodology: Engineering Precision Therapy
The research involved multiple innovative steps to develop the therapeutic platform. MSC-derived EVs were engineered to overexpress CHIP, an E3 ubiquitin ligase critical for degrading fibrosis-related proteins such as Smad2/3. The vesicles were further modified with SPION, enabling them to be guided magnetically to the kidneys.
The team employed both in vitro and in vivo models to assess the efficacy of these SPION-EVs. In vitro, renal tubular cells were treated to observe reductions in fibrosis markers. In vivo, rat models of renal fibrosis, including those induced by unilateral ureteral obstruction (UUO) and diabetic kidney disease (DKD), were used to evaluate renal targeting, anti-fibrotic effects, and overall safety.
Results: A Closer Look at the Efficacy of SPION-EVs
The study yielded remarkable findings, showcasing the potential of SPION-decorated MSC-EVs with CHIP overexpression as a therapeutic platform for renal fibrosis.
Enhanced Targeting and Delivery
SPION-EVs demonstrated superior renal targeting compared to conventional MSC-EVs. Using a magnetic field, the vesicles were guided to the kidneys, as evidenced by fluorescence imaging. In unilateral ureteral obstruction (UUO) rat models, SPION-EVs accumulated significantly in injured renal tissue, as confirmed by CM-DIR fluorescence signals and western blot analysis, which revealed elevated CHIP expression in treated kidneys.
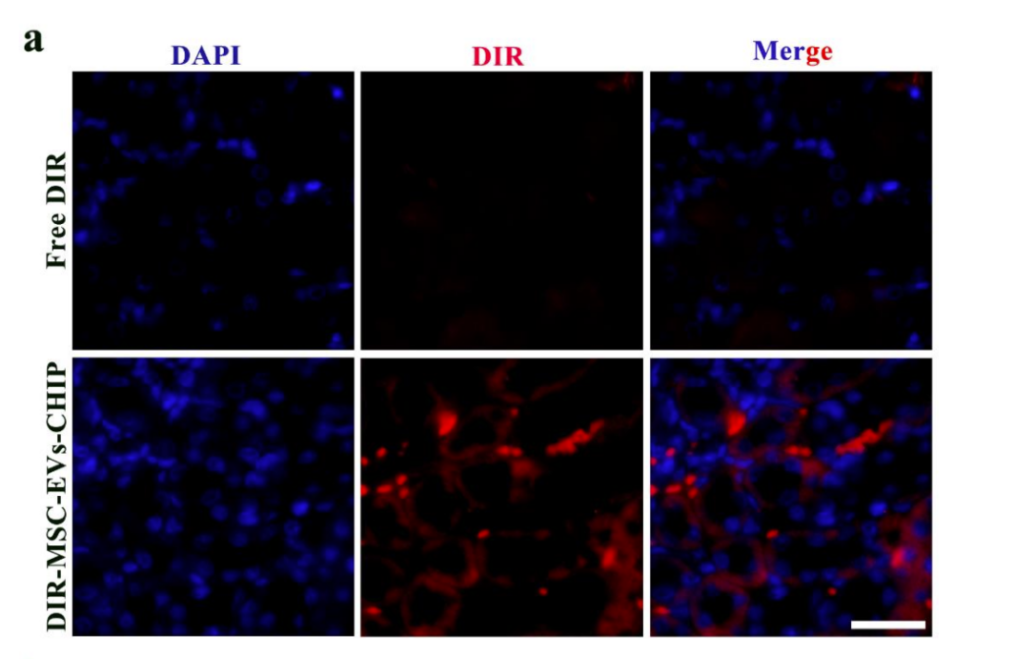
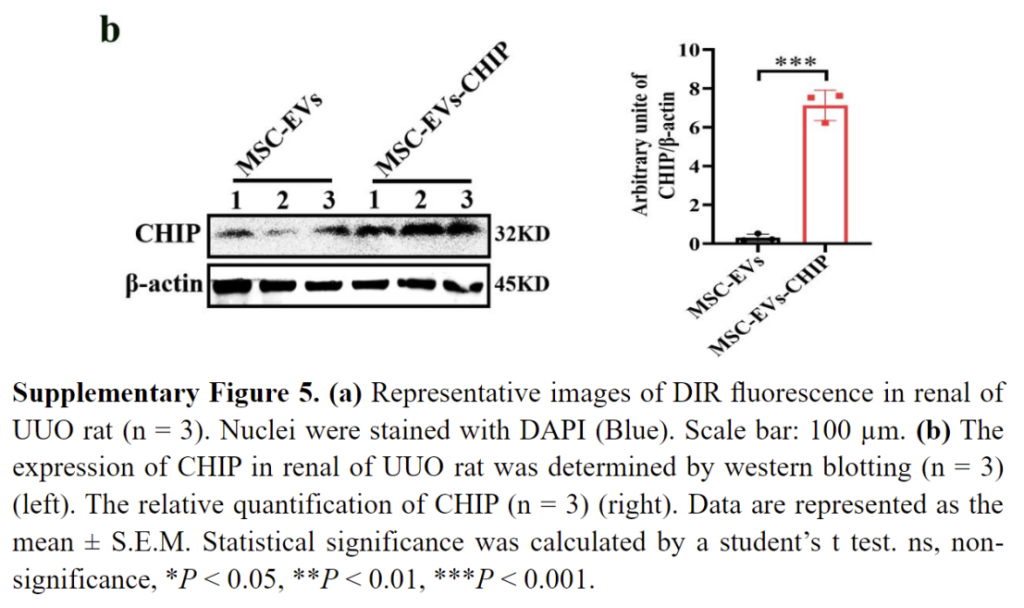
Reduction of Fibrotic Markers
SPION-EVs effectively reduced fibrosis-related protein levels. Immunohistochemistry and western blotting showed marked decreases in α-SMA, fibronectin, and collagen I. In histological analyses, Sirius red and Masson trichrome staining revealed that SPION-EVs significantly reduced interstitial collagen deposition compared to untreated and MSC-EV-treated groups.
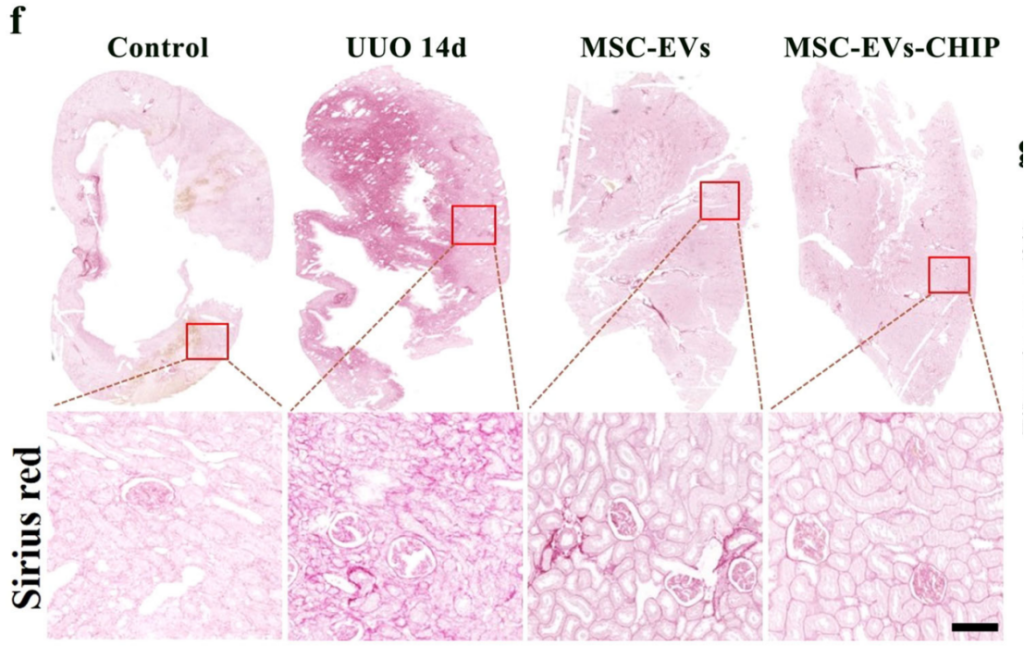
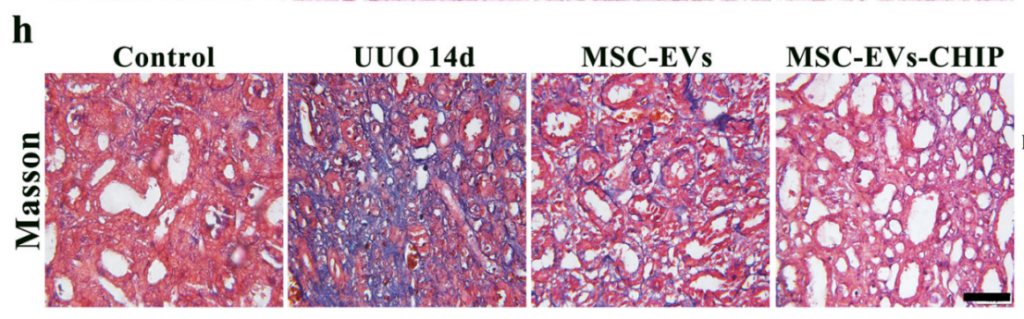
Improved Renal Function
Biochemical markers of renal function, including creatinine and urea nitrogen levels, were significantly improved in SPION-EV-treated rats. This improvement reflected the enhanced clearance of metabolic waste, indicative of better kidney function.
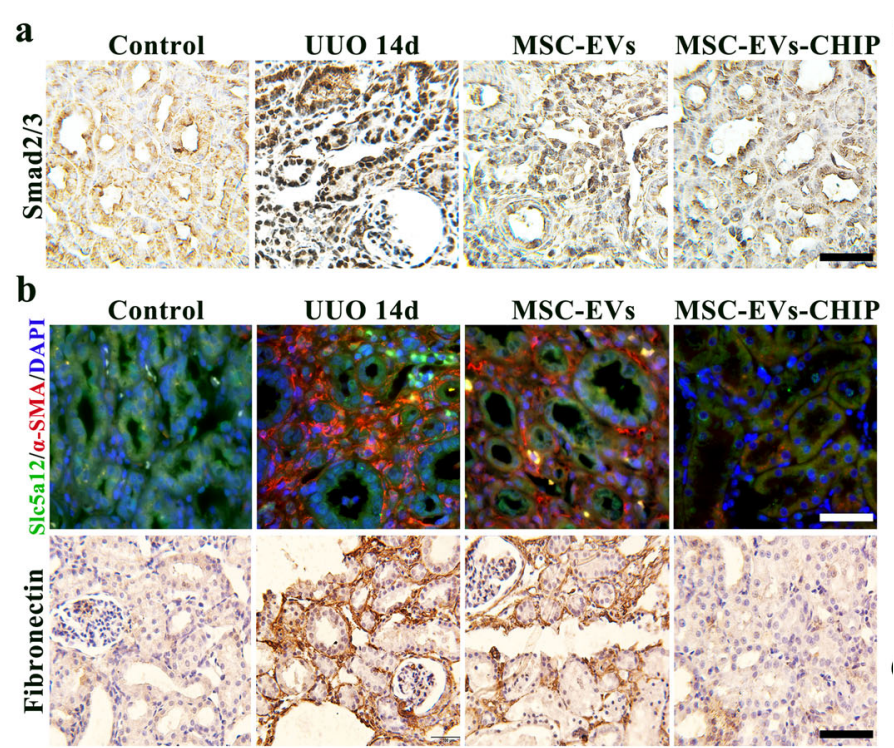
Smad2/3 Ubiquitination and Degradation
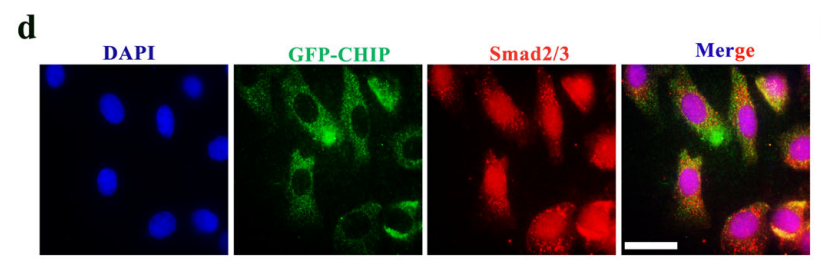
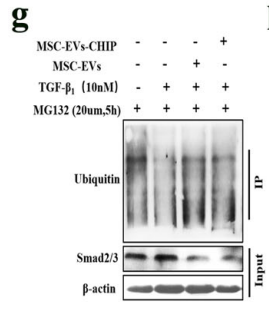
The therapeutic mechanism of SPION-EVs centers on promoting the ubiquitination and degradation of Smad2/3, a key protein implicated in renal fibrosis. Confocal microscopy confirmed the co-localization of CHIP with Smad2/3, and co-immunoprecipitation demonstrated increased Smad2/3 ubiquitination in renal tubular cells treated with SPION-EVs. Consequently, Smad2/3 expression in the nucleus was markedly reduced, mitigating its role in fibrosis progression.
Inflammation Mitigation
SPION-EVs also exhibited potent anti-inflammatory effects. Treated rats showed reduced levels of inflammatory cytokines, including IL-6, IL-1β, and TNF-α. CD66+ neutrophil infiltration was significantly lower in SPION-EV-treated kidneys, as demonstrated by immunofluorescence staining.
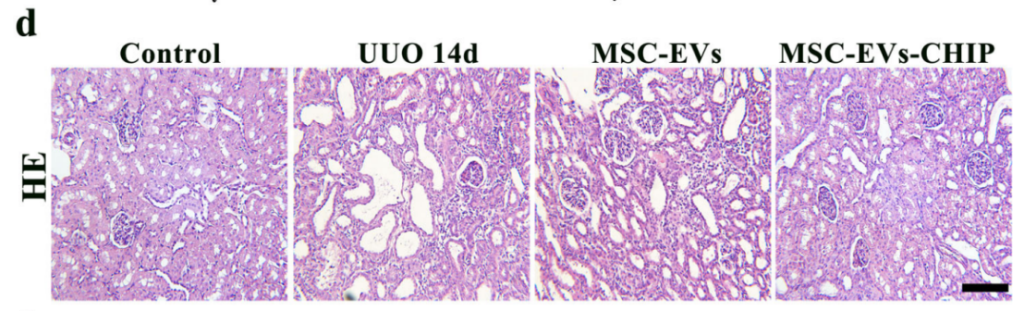
Histological Recovery
Histological examinations highlighted the structural recovery of kidney tissues. Hematoxylin and eosin staining revealed diminished tubular vacuolar degeneration and restoration of normal renal architecture. In SPION-EV-treated rats, the damage score dropped significantly from 70% (untreated) to 18%.
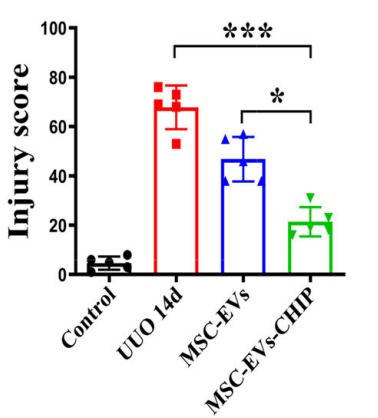
Superior to MSC-EVs Alone
Compared to standard MSC-EVs, SPION-EVs consistently outperformed in all metrics, including fibrosis reduction, renal function improvement, and inflammation control. These results underline the added value of SPION decoration for targeted delivery and enhanced therapeutic effects.
By addressing both the localization and efficacy challenges of renal fibrosis treatments, SPION-EVs emerge as a groundbreaking approach, combining innovative nanotechnology with biological engineering for superior outcomes.
Conclusion: A New Frontier in CKD Treatment
This research underscores the transformative potential of SPION-decorated MSC-EVs in renal fibrosis therapy. By enhancing the delivery and efficacy of CHIP, this platform addresses both the therapeutic and targeting challenges that have historically limited CKD treatments.
The implications for clinical applications are profound. With further refinement, SPION-EVs could become a cornerstone in the management of renal fibrosis, offering hope to millions worldwide. However, the study acknowledges limitations, including the need for long-term safety evaluations and the scalability of the production process. Future research will focus on optimizing these parameters and exploring the platform’s application in other fibrotic conditions.
Through the convergence of nanotechnology and regenerative medicine, this innovative approach represents a leap forward in the fight against chronic kidney disease.
Reference:
Ji, Cheng, et al. “Engineered extracellular vesicle-encapsulated CHIP as novel nanotherapeutics for treatment of renal fibrosis.” NPJ Regenerative Medicine 9.1 (2024): 3.
