Scientists have successfully utilized a hepatocyte-specific base editing strategy via dual-AAV delivery to achieve a remarkable reduction in blood lipid levels in mice by targeting ANGPTL3.
Background — Targeting ANGPTL3: A Game-Changer in Lipid Management
Cardiovascular disease (CVD) remains one of the leading causes of mortality worldwide, with hypercholesterolemia as a primary risk factor. Elevated low-density lipoprotein cholesterol (LDL-C) and very-low-density lipoprotein cholesterol (VLDL-C) contribute to the progression of CVD. While existing lipid-lowering therapies such as statins and PCSK9 inhibitors provide significant cardiovascular risk reductions, many patients fail to achieve adequate LDL-C control due to drug intolerance or insufficient response.
Angiopoietin-like protein 3 (ANGPTL3) has emerged as a novel therapeutic target because of its critical role in lipid metabolism. It acts as an endogenous inhibitor of lipoprotein lipase (LPL), thereby regulating triglyceride (TG) and cholesterol levels. Therapies targeting ANGPTL3, including monoclonal antibodies (e.g., evinacumab) and antisense oligonucleotides (ASOs), have shown promise in reducing blood lipid levels. However, these approaches require repeated administrations, highlighting the need for a long-lasting, efficient therapeutic option.
Research Aim & Objectives — Developing a Hepatocyte-Specific Gene Editing Approach
In a study led by Yuanbojiao Zuo et al., published in Cell & Bioscience (2023), researchers explored a novel hepatocyte-specific gene editing approach. Their aim was to develop a one-time treatment for hypercholesterolemia by using adeno-associated virus (AAV)-mediated cytosine base editing to disrupt ANGPTL3 expression in mice. The study sought to overcome the limitations of existing therapies, focusing on efficiency, specificity, and safety.
Research Methods — Innovating AAV Delivery for Precision Editing
The team employed a dual-AAV9 delivery system with the following key components:
- Base Editor System: The cytosine base editor (AncBE4max) was split into two parts using an intein split strategy to fit into the packaging limits of AAV vectors.
- Hepatocyte-Specific Promoter: The human alpha-1-antitrypsin (hAAT) promoter ensured liver-specific expression of the base editor.
- Editing Target: The ANGPTL3 gene was disrupted by introducing a premature stop codon (Q135X), silencing its expression.
The approach avoided DNA double-strand breaks, reducing the risk of off-target effects and improving the safety profile.
Results — Promising Outcomes for Lipid Reduction
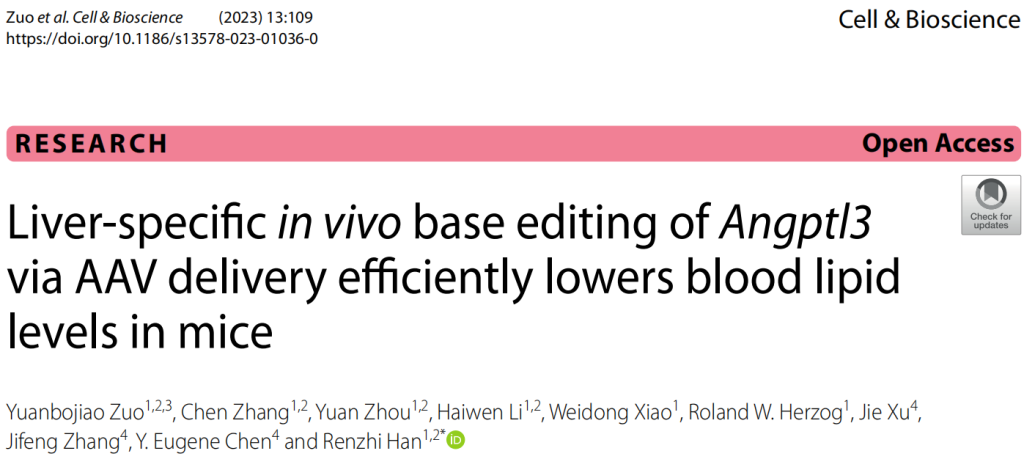
- High Editing Efficiency:
- The dual-AAV9 system achieved a genome editing efficiency of 63.3 ± 2.3% in mouse liver tissues, as determined by sequencing the ANGPTL3 locus.
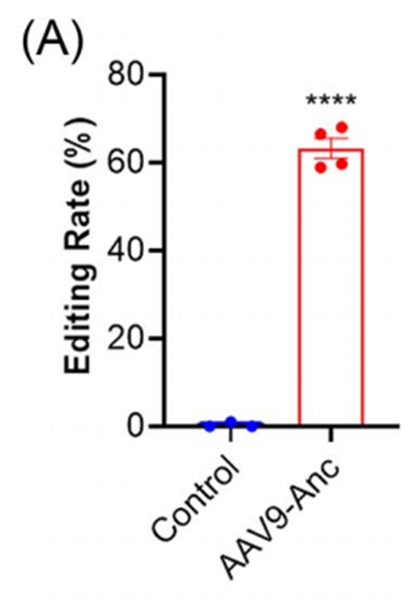
- Near-Complete ANGPTL3 Protein Knockdown:
- Within 1–4 weeks post-injection, circulating ANGPTL3 protein levels were significantly reduced. At 4 weeks, protein levels were almost undetectable.
- Pre-treatment levels: 161.2 ± 9.2 ng/mL
- Post-treatment (1 week): 92.9 ± 6.8 ng/mL
- Post-treatment (2–4 weeks): Nearly undetectable
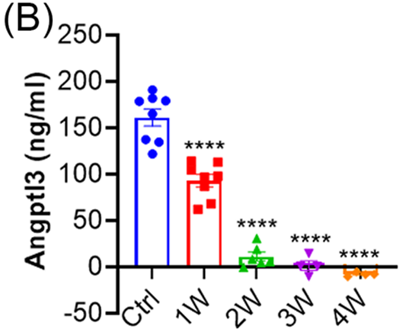
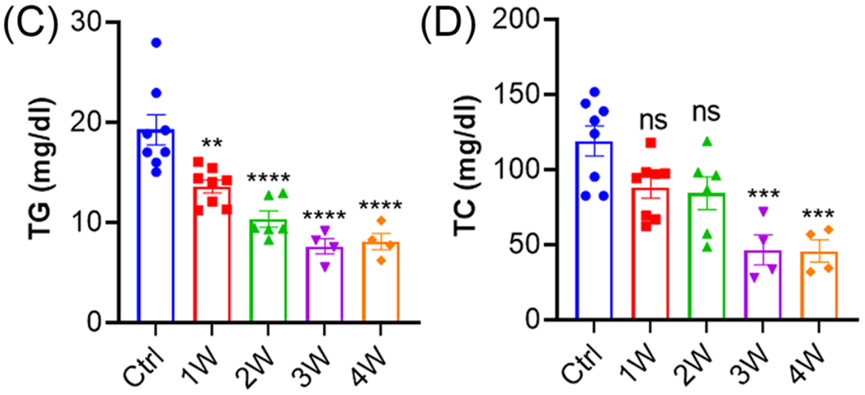
- Significant Reductions in Blood Lipids:
- Serum triglyceride (TG) levels decreased from 19.3 ± 1.5 mg/dL to 8.1 ± 0.8 mg/dL at 4 weeks post-treatment, representing a 58% reduction.
- Total cholesterol (TC) levels decreased by 61%, from pre-treatment levels to 4 weeks after injection.
- Minimal Off-Target Effects:
- Liver-specific expression of the base editor ensured that editing was confined to hepatocytes. Analysis of non-liver tissues (e.g., heart, kidney, skeletal muscle) showed undetectable off-target editing.
- No Liver Toxicity:
- Serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) remained stable, and histological examination revealed no signs of liver inflammation or immune cell infiltration.
These findings underscore the potential of AAV-mediated ANGPTL3 base editing as a safe and effective treatment for hypercholesterolemia.
Summary — Towards a Breakthrough in Cardiovascular Therapy
This study demonstrates the potential of AAV-mediated ANGPTL3 base editing as a transformative therapy for hypercholesterolemia and associated cardiovascular risks. By achieving a high editing efficiency of 63.3%, nearly complete suppression of ANGPTL3 protein, and substantial reductions in triglycerides and total cholesterol, the research highlights the promise of a one-time gene-editing approach for lipid management. Unlike existing treatments requiring repeated administrations, this strategy offers a long-term solution with liver-specific precision, as evidenced by the absence of detectable off-target effects in non-liver tissues. Furthermore, the therapy showed no signs of liver toxicity, with stable liver enzyme levels and no histological abnormalities, underscoring its safety profile.
Despite these promising results, several challenges remain to be addressed. One major concern is the prolonged expression of the editing machinery delivered via AAV vectors, which could lead to an accumulation of off-target effects or immune responses. Researchers suggest that future studies should focus on developing methods to achieve temporal control over the expression of base editors, potentially limiting their activity to the necessary duration for efficient gene editing. Non-viral delivery methods, such as lipid nanoparticles (LNPs), are proposed as alternative strategies to overcome the risks associated with AAV vectors. These approaches may enhance safety, improve scalability, and offer greater flexibility for clinical applications. Another aspect requiring attention is the scalability and tissue specificity of emerging delivery methods like LNPs or viral-like particles (VLPs), as well as their long-term stability and feasibility.
Reference:
Zuo, Yuanbojiao, et al. “Liver-specific in vivo base editing of Angptl3 via AAV delivery efficiently lowers blood lipid levels in mice.” Cell & Bioscience 13.1 (2023): 109.
