Introduction: A Persistent Challenge in Diabetic Wound Treatment
Diabetic wounds, affecting nearly 25% of patients with diabetes, have long posed a challenge to modern medicine due to their susceptibility to infection, chronic inflammation, and impaired healing. Traditional approaches, such as debridement and bioengineered tissue applications, have often fallen short in addressing the complex microenvironments of these wounds. Recently, microneedle technology has emerged as a transformative innovation, offering precision drug delivery and minimal invasiveness. This research tackles the intricate issue of diabetic wound biofilms, focusing on a multifunctional microneedle bandage that not only eradicates biofilms but also promotes healing through dual-directional reactive species regulation.
Objective of the Study: A Collaborative Push for Change

The study, published in Nature Communications, represents a significant collaborative effort by researchers from institutions including Sun Yat-sen University and Nanyang Technological University. Key contributors like Li Yang, Dan Zhang, and Xiaowei Zeng led the research, which centers on a novel SeC@PA microneedle bandage. The study’s primary goal was to address the limitations of existing diabetic wound treatments by creating a self-enhancing therapy capable of disrupting biofilms, reducing inflammation, and accelerating tissue regeneration.
Methodology: Engineering a Multifunctional Solution
The theoretical foundation of this breakthrough lies in leveraging the unique biofilm microenvironment to enhance the therapeutic efficacy of photodynamic therapy (PDT). The microneedle bandage employs dopamine-coated hybrid nanoparticles containing selenium and chlorin e6 (SeC@PA), enabling bidirectional regulation of reactive oxygen and nitrogen species.
The research design involved the development of a microneedle bandage system capable of penetrating wound barriers and delivering SeC@PA nanoparticles directly to affected tissue. Methods of data collection included in vitro analyses to evaluate the reactive species regulation and anti-biofilm efficacy, alongside in vivo studies using diabetic mouse models to monitor wound healing outcomes and macrophage activity.
Results: Groundbreaking Efficacy of SeC@PA Microneedles
The study highlights the remarkable capabilities of SeC@PA microneedles in eradicating biofilms, modulating inflammation, and accelerating wound healing in diabetic models. The following results were supported by robust data and visual evidence from the experiments.
Biofilm Eradication Through Reactive Species (RS) Storm
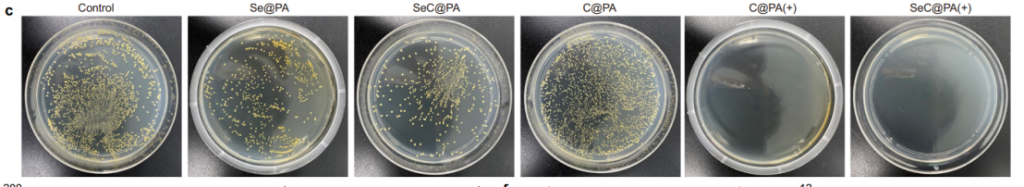
SeC@PA microneedles achieved superior biofilm clearance by depleting glutathione (GSH) and amplifying RS levels within biofilms. Figure 3a showcases live/dead staining images of Staphylococcus aureus biofilms, revealing a significant increase in dead bacterial cells after treatment with SeC@PA microneedles.
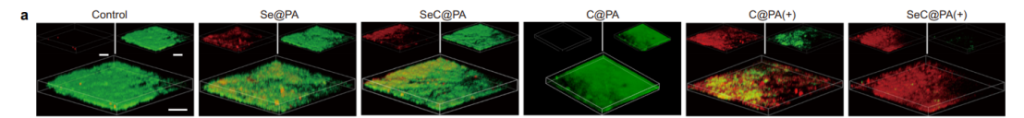
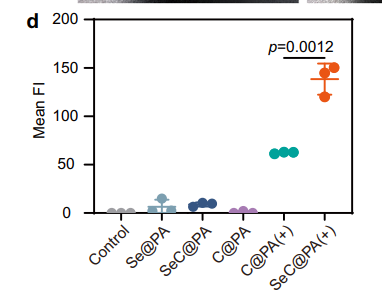
Quantitative analysis of RS generation confirmed a increase in ROS levels, while bacterial colony counts demonstrated a reduction.
Dual Regulation of Reactive Species
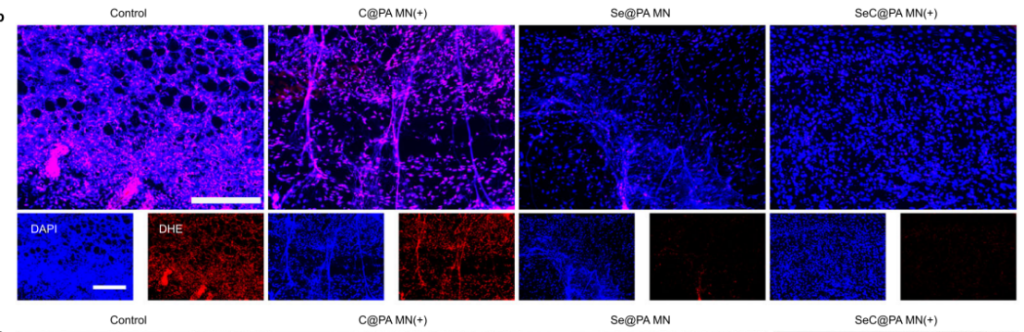
The microneedles exhibited bidirectional RS regulation, amplifying RS in high-GSH biofilms and scavenging RS in low-GSH wound tissues. Figure 2e highlights this dual functionality, showing increased RS intensity in biofilms and reduced RS in tissues.
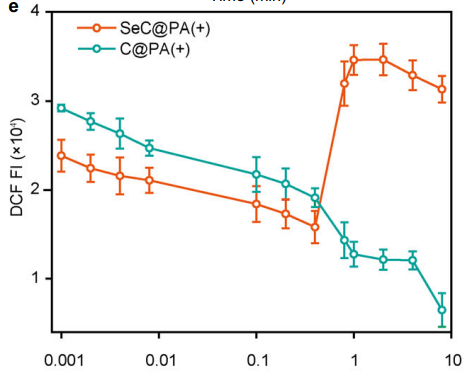
Fluorescence assays of RS in tissues indicated a decrease in oxidative stress after SeC@PA treatment.
Accelerated Wound Healing
Wound healing rates significantly improved with SeC@PA microneedles. It is displayed that photographic evidence of wounds treated with microneedles achieving 95% closure within 16 days.
Macrophage Polarization and Inflammation Modulation
The microneedles promoted macrophage polarization to the M2 phenotype, critical for anti-inflammatory responses and wound repair. Flow cytometry data showed a increase in M2 macrophages, while Western blot analysis revealed a significant upregulation of anti-inflammatory cytokines such as IL-10. In contrast, pro-inflammatory markers like TNF-α decreased markedly.
Efficient Drug Delivery
The microneedle system demonstrated superior penetration capabilities compared to traditional nanoparticle solutions. Figure 7e and Figure 7f illustrate the deeper penetration of microneedles into biofilm-covered wounds, bypassing the barriers that typically hinder drug delivery.
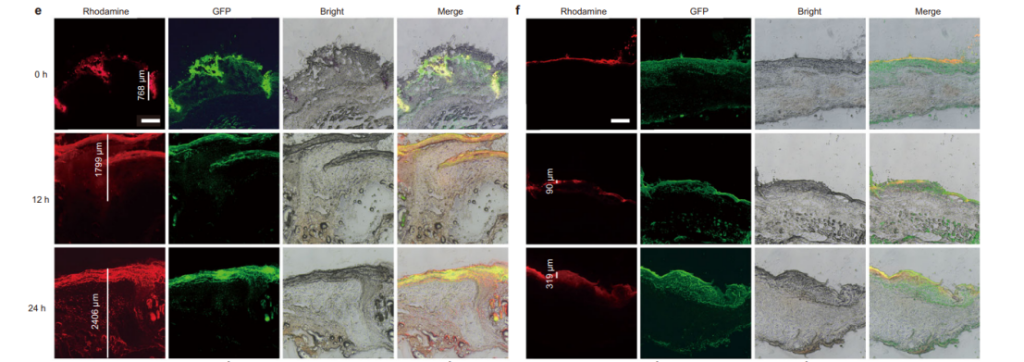
These results, supported by comprehensive visual data, underscore the transformative potential of SeC@PA microneedles in diabetic wound therapy, offering a targeted, multifaceted approach to chronic wound management.
Conclusion: A New Horizon for Chronic Wound Management
This research represents a milestone in diabetic wound therapy, showcasing the potential of microneedle technology to overcome longstanding challenges in biofilm management and tissue regeneration. The SeC@PA microneedle bandage’s ability to regulate reactive species, mitigate inflammation, and enhance wound healing positions it as a promising candidate for clinical translation.
However, limitations such as the need for optimized delivery protocols and scalability for human application were noted. Future research should focus on long-term efficacy, diverse wound environments, and large-scale clinical trials to validate this therapy’s broader applicability.
By bridging innovative engineering with medical need, this study not only advances diabetic wound treatment but also highlights the broader potential of microneedle-based systems in addressing complex medical conditions.
Reference:
Yang, Li, et al. “Biofilm microenvironment triggered self-enhancing photodynamic immunomodulatory microneedle for diabetic wound therapy.” Nature Communications 14.1 (2023): 7658.
