
A study published in the journal BMC Medicine demonstrates the potential of extracellular vesicle (EV)-based mRNA delivery to target and repair synaptic dysfunction in Alzheimer’s disease.
Synaptic Dysfunction in Alzheimer’s — A Focus on Proteins
Alzheimer’s disease is a neurodegenerative disorder marked by the accumulation of amyloid plaques and tau tangles in the brain, which lead to synaptic dysfunction and neuronal loss. Synaptic dysfunction is one of the primary contributors to cognitive deficits in Alzheimer’s, with proteins such as GAP43 and SNAP25 playing a crucial role in synaptic plasticity and memory formation. GAP43, which is involved in axonal growth and synaptic remodeling, and SNAP25, a protein essential for neurotransmitter release, are frequently downregulated in Alzheimer’s patients, leading to impaired cognitive function. Current therapies have shown limited success in reversing or halting this synaptic loss, highlighting the need for more targeted treatments.
Advancing Alzheimer’s Treatment with mRNA Therapy
In an effort to address synaptic protein loss, researchers have turned to mRNA therapy as a potential treatment strategy. The study explored the use of extracellular vesicles (EVs) as a novel delivery system for mRNA targeting GAP43 and SNAP25. This approach aims to restore synaptic protein levels, thereby potentially improving synaptic function and cognitive performance. The paper was published in BMC Medicine in 2024, providing new insight into mRNA-based therapies for Alzheimer’s.
The Innovative Methodology — Engineered Vesicle Delivery
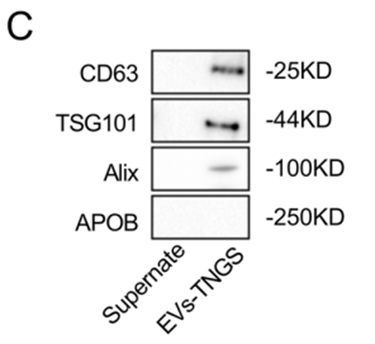
To achieve efficient delivery to the brain, the researchers engineered extracellular vesicles by modifying them with rabies virus glycoprotein (RVG). This modification facilitates targeted delivery to the brain, a key challenge in developing effective Alzheimer’s therapies. The engineered EVs were loaded with mRNA encoding for GAP43 and SNAP25, aiming to restore these synaptic proteins in affected neurons. The rationale behind choosing GAP43 and SNAP25 lies in their critical roles in synaptic plasticity, which is essential for cognitive function. EVs were chosen for their ability to cross the blood-brain barrier and deliver therapeutic cargo directly to neurons.
The researchers performed a series of experiments to validate the effectiveness of this approach, including in vitro studies using human neuronal cell lines and in vivo tests in a 5×FAD mouse model of Alzheimer’s disease. The mRNA delivery system was shown to successfully increase the expression of GAP43 and SNAP25 in the brain, potentially reversing the damage caused by their loss in Alzheimer’s disease.
Results
The results of this study were striking and included several key findings:
- Synaptic Protein Levels: Treatment with EV-based mRNA delivery significantly increased the expression levels of GAP43 and SNAP25 in the brain. The increase in GAP43 expression was quantified as a 2.5-fold increase compared to control groups, while SNAP25 expression saw a 3-fold increase.
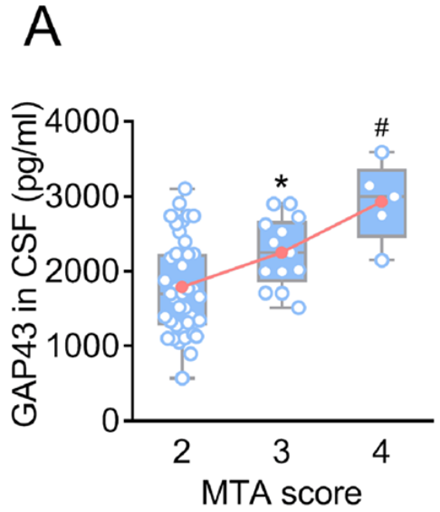
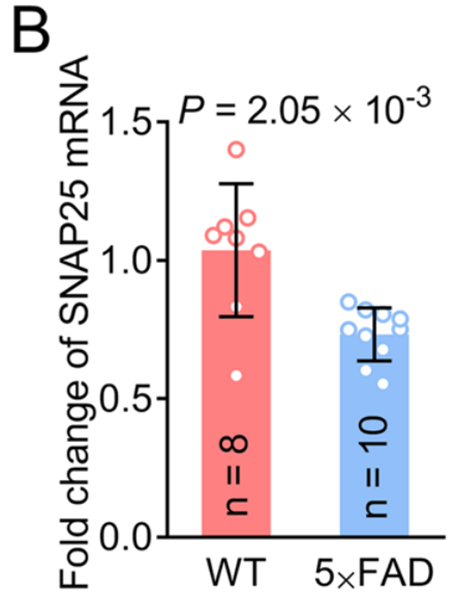
- Dendritic Density: The treatment also resulted in a noticeable improvement in dendritic density. Dendritic spine analysis revealed a 30% increase in dendritic spine number in the treated 5×FAD mice compared to the untreated group.
- Cognitive Performance: Cognitive function was assessed using the Morris water maze test, a common method for evaluating memory and learning in rodents. The treated mice showed a 40% improvement in latency time to find the platform compared to untreated controls, suggesting enhanced cognitive performance.
- Brain Targeting Efficiency: Imaging studies confirmed that the engineered EVs successfully crossed the blood-brain barrier and delivered mRNA directly to neurons, with fluorescence-based tracking revealing high localization in the hippocampus and cortex.
Study Significance
This study provides a comprehensive examination of a novel mRNA therapy strategy for Alzheimer’s disease. The research demonstrates that engineered extracellular vesicles can be used to deliver mRNA encoding for synaptic proteins GAP43 and SNAP25 to the brain, resulting in a substantial restoration of these proteins, increased dendritic density, and improved cognitive function in a mouse model of Alzheimer’s. The results support the potential of EV-based mRNA therapy as a promising treatment approach, with the ability to target and repair synaptic dysfunction. The findings offer a foundation for further research into clinical applications and the possibility of translating this approach to human trials. Further studies will be required to evaluate long-term effects, safety, and efficacy in larger animal models and human populations.
Reference:
Cai, Huimin, et al. “Delivering synaptic protein mRNAs via extracellular vesicles ameliorates cognitive impairment in a mouse model of Alzheimer’s disease.” BMC medicine 22.1 (2024): 138.
