Pioneering Advances in Chondrosarcoma Treatment
Chondrosarcoma, a malignant tumor arising from cartilage, has long presented a formidable challenge in oncology due to its resistance to conventional chemotherapy and radiotherapy. Factors such as poor vascularization, dense extracellular matrix, and hypoxic microenvironment make it inherently difficult to treat. Historically, surgical resection has been the primary curative method, but its application is often limited by anatomical constraints and the risk of severe functional impairment. Recently, innovative strategies, including the use of nanoparticles in combination with radiation therapy, have emerged as a beacon of hope. By enhancing radiosensitivity, nanoparticles offer a new dimension in addressing this resistant tumor type, paving the way for breakthroughs in cancer treatment.
Targeting the Toughest Tumors: Research Objectives and Vision

Led by a multidisciplinary team comprising Mihaela Tudor, Roxana Cristina Popescu, and Diana Iulia Savu from institutions such as the Horia Hulubei National Institute for R&D in Physics and Nuclear Engineering, the University of Bucharest, and others, this study represents a significant milestone. Published in Scientific Reports in 2023, the research explores the synergistic effects of polyethylene glycol-encapsulated iron oxide nanoparticles loaded with doxorubicin (IONPDOX) and high-linear energy transfer (LET) carbon ion radiation. The primary objective was to assess the potential of these nanoparticles to overcome chondrosarcoma’s notorious resistance by enhancing DNA damage and reducing cell survival rates.
Innovative Methodologies: A Multi-Pronged Approach
The study employed a robust experimental design centered on SW1353 chondrosarcoma cells. Researchers first treated cells with IONPDOX to ensure nanoparticle uptake and retention, a critical step for effective radiosensitization. Radiation therapy was conducted using both carbon ions, known for their superior biological effectiveness, and conventional X-rays.
To measure treatment efficacy, a variety of data collection methods were utilized. Cell survival assays quantified the cytotoxic effects of the combined treatments, while micronucleus assays provided insights into DNA damage. Hyperspectral imaging, coupled with enhanced dark-field microscopy, offered a novel means to analyze alterations in cell morphology and nuclear spectral profiles.
Unveiling Groundbreaking Results
The study’s findings reveal the substantial potential of nanoparticle-mediated radiosensitization in enhancing chondrosarcoma treatment outcomes. The integration of polyethylene glycol-encapsulated iron oxide nanoparticles loaded with doxorubicin (IONPDOX) demonstrated a notable ability to amplify the effects of both carbon ion and X-ray irradiation.
Enhanced Cytotoxicity and Reduced Cell Survival
The cytotoxic effects of IONPDOX were evident in cell survival assays. The survival fraction (SF) of SW1353 cells decreased significantly when treated with IONPDOX in combination with radiation. For instance, the survival fraction at 1 Gy decreased to 0.52 ± 0.19 when treated with IONPDOX alone.
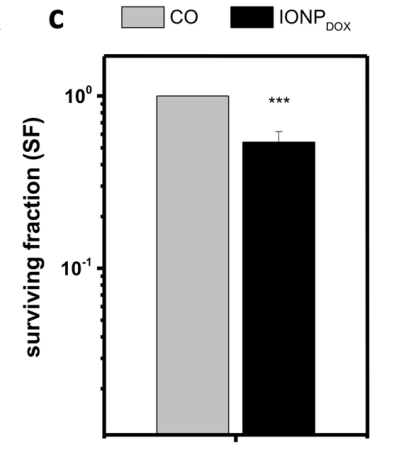
When combined with radiation, the reduction was more pronounced, particularly with carbon ions, which exhibited higher linear energy transfer (LET). Statistical analysis confirmed a dose-modifying factor (DMF) of 1.2 ± 0.1 for carbon ion radiation, compared to 1.05 ± 0.03 for X-ray irradiation. This indicates that carbon ions, in conjunction with IONPDOX, provide superior sensitization compared to X-rays.
Increased DNA Damage through Micronucleus Formation
Micronucleus assays provided further insights into the genotoxic effects of the treatment. The presence of micronuclei, a marker of DNA damage, was significantly higher in cells exposed to combined treatments. For instance, IONPDOX with 0.5 Gy carbon ion radiation induced a substantial increase in micronuclei compared to radiation alone (p = 0.0056). A similar trend was observed with X-ray irradiation, where IONPDOX amplified the DNA damage, particularly at lower doses such as 0.5 Gy (p = 0.016). These results underscore the role of IONPDOX in exacerbating radiation-induced DNA damage in chondrosarcoma cells.
Morphological Alterations and Hyperspectral Analysis
The study utilized enhanced dark-field microscopy paired with hyperspectral imaging (HSI) to evaluate cellular and nuclear changes. Morphological analysis revealed significant alterations in cell structure and density post-treatment, correlating with reduced clonogenic survival. Hyperspectral imaging provided detailed spectral profiles of cell nuclei, highlighting distinct changes induced by the combined treatment. Untreated cells exhibited characteristic peaks at 424 nm and 445 nm, with a valley at 436 nm. These spectral features were drastically altered following IONPDOX and radiation treatment. For example, carbon ion irradiation shifted the 424 nm peak to 430 nm and caused the disappearance of the 445 nm peak, indicating severe nuclear alterations.
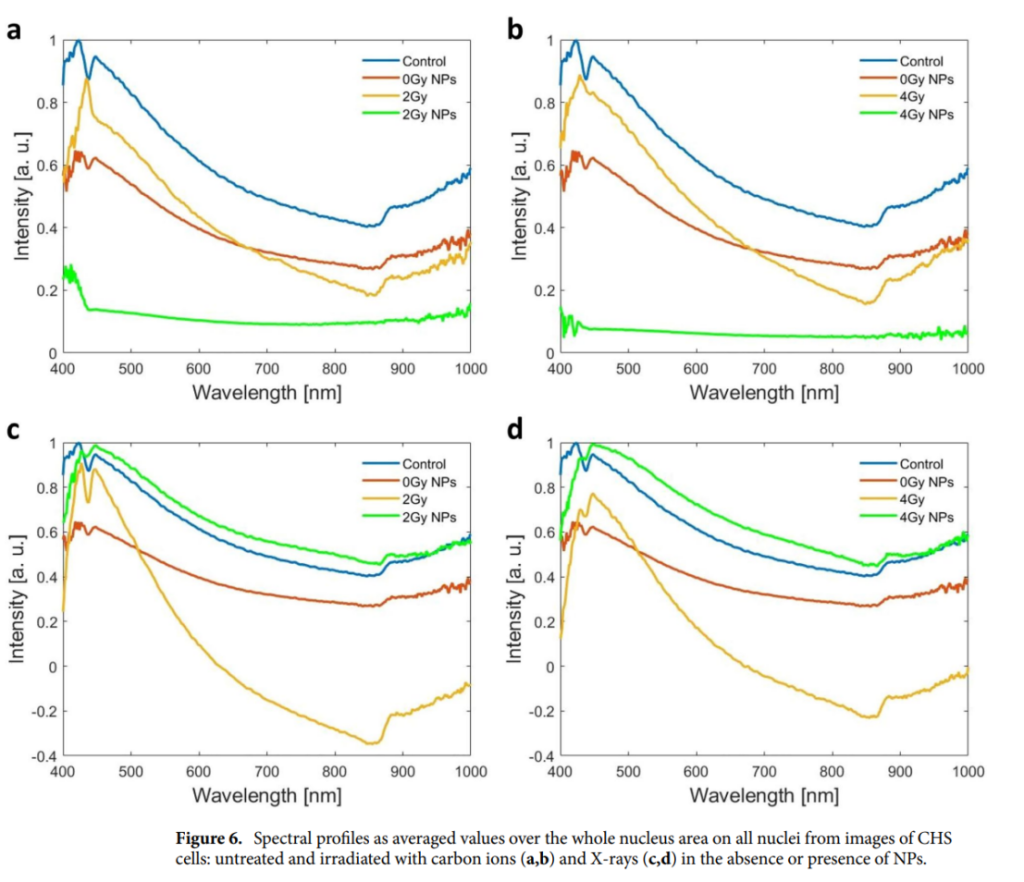
Synergistic Effects of IONPDOX and Radiation
The combined treatment demonstrated a clear synergistic effect, with IONPDOX amplifying the radiation-induced oxidative stress and DNA damage. The presence of nanoparticles not only heightened the cytotoxic impact of radiation but also reduced cell viability in a dose-dependent manner. Carbon ion radiation, known for its precise energy deposition, achieved better radiosensitization outcomes than X-rays when paired with IONPDOX.
In summary, the results highlight the promising role of nanoparticle-mediated radiosensitization in overcoming the inherent resistance of chondrosarcoma cells to traditional therapies. These findings lay the foundation for further exploration into nanoparticle-based combination treatments to improve therapeutic efficacy while minimizing adverse effects.
Implications and Future Horizons
This research underscores the transformative potential of nanoparticle-mediated radiosensitization in addressing chondrosarcoma’s treatment challenges. By improving radiation efficacy and reducing systemic toxicity, this approach could redefine therapeutic standards for resistant tumors.
However, the study acknowledges limitations, including the modest radiosensitization effects observed and the need for further in vivo validation. Future research should explore optimizing nanoparticle formulations and delivery mechanisms to enhance therapeutic outcomes further.
In conclusion, this pioneering study heralds a new era in cancer treatment, where advanced nanotechnology and precision radiotherapy converge to tackle the toughest challenges. With continued innovation and collaboration, the vision of improved outcomes for chondrosarcoma patients may soon become a reality.
Reference:
Tudor, Mihaela, et al. “In vitro hyperspectral biomarkers of human chondrosarcoma cells in nanoparticle-mediated radiosensitization using carbon ions.” Scientific Reports 13.1 (2023): 14878.
