
Introduction: The Evolution of Chemotherapy and the Rise of Resistance in Breast Cancer
Chemotherapy has long been a cornerstone in the treatment of aggressive breast cancers, including triple-negative breast cancer (TNBC), where hormone receptors such as estrogen, progesterone, and HER2 are absent. This treatment, which involves the use of cytotoxic drugs to kill rapidly dividing cancer cells, has significantly improved survival rates over the past few decades. However, the emergence of chemoresistance remains a formidable challenge in oncology, with about 25% of breast cancer patients experiencing metastasis even after chemotherapy. This resistance often results from complex interactions within the tumor microenvironment (TME), particularly involving tumor-associated macrophages (TAMs).
In recent years, scientists have begun to focus on TAMs as a key player in the progression of chemotherapy-resistant tumors. These macrophages, which are a major component of the TME, can be polarized into two types: M1 and M2. While M1 macrophages have antitumor properties, M2 macrophages, which are prevalent in breast cancer, enhance tumor progression by promoting inflammation, invasion, and metastasis. New research has unveiled a critical interaction between TAMs and a protein known as extracellular heat shock protein 70 (HSP70). This protein, which is typically associated with cellular stress, appears to play a significant role in modulating the behavior of TAMs, particularly following chemotherapy. Understanding the relationship between HSP70 and TAMs could provide valuable insights into overcoming chemotherapy resistance.
Research Objectives: Understanding the Role of HSP70 and TAMs in Chemoresistance
A groundbreaking study led by Mio Yamaguchi-Tanaka and colleagues at Tohoku University, Japan, published in Cancers (2023), addresses the question of how chemotherapy influences the behavior of TAMs, particularly through the secretion of extracellular HSP70. The research aims to clarify whether chemotherapy-induced HSP70, secreted by breast cancer cells, enhances the pro-tumorigenic functions of TAMs, thus contributing to chemoresistance. This study is significant as it offers a new perspective on the molecular mechanisms that drive chemotherapy resistance and suggests potential therapeutic avenues to improve patient outcomes. By exploring the interaction between TAMs, HSP70, and transforming growth factor (TGF)-β in the TME, the research aims to identify novel targets for therapeutic intervention.
Methodology: Exploring the Mechanisms Behind Chemoresistance
The study’s theoretical framework revolves around the concept that chemotherapy-induced extracellular HSP70 interacts with TAMs to promote tumor growth. Using in vitro experiments, the research team tested the hypothesis that HSP70 secretion by chemotherapy-treated breast cancer cells enhances the tumor-promoting activity of TAMs. The study specifically focused on how HSP70 influences TAM function by regulating cytokine expression, particularly TGF-β, in breast cancer cells.
To test this hypothesis, the research employed a robust experimental design, including co-culture assays with human triple-negative breast cancer (TNBC) cell lines (MDA-MB-231 and MDA-MB-453) and THP-1-derived macrophages. The team used epirubicin (EPI), a chemotherapy drug, to treat the breast cancer cells and examine its effects on the secretion of extracellular HSP70. The study also involved immunohistochemical analysis of breast cancer tissues to assess the correlation between HSP70 expression and macrophage infiltration. Data collection methods included Western blotting, fluorescence immunocytochemistry, and small interfering RNA (siRNA)-mediated knockdown of HSP70, allowing the team to assess changes in macrophage polarization and the expression of pro-tumorigenic markers.
Results: Unraveling the Impact of HSP70 on TAM-Mediated Tumor Progression
The study’s findings revealed compelling data on the interaction between chemotherapy-induced extracellular HSP70 and tumor-associated macrophages (TAMs) in the context of breast cancer. Chemotherapy, specifically epirubicin (EPI), was shown to significantly increase the secretion of HSP70 from breast cancer cells, particularly in small extracellular vesicles (sEVs).
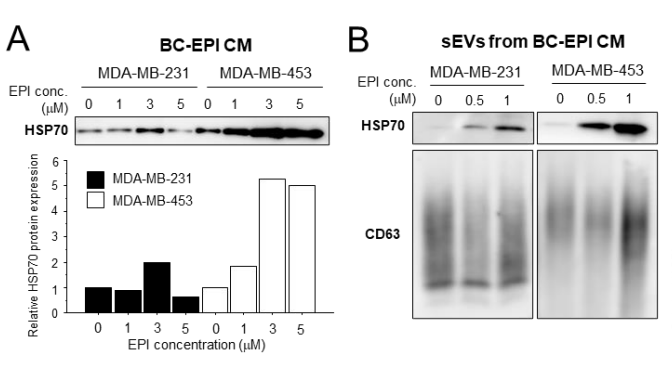
In the in vitro experiments, MDA-MB-231 and MDA-MB-453 breast cancer cells treated with EPI exhibited a clear increase in HSP70 levels within the conditioned medium (CM) compared to untreated cells.
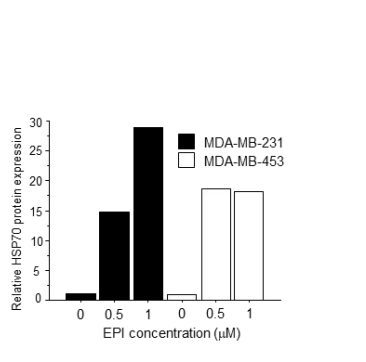
Notably, HSP70 was enriched in the sEVs isolated from the CM, with the peak concentration of HSP70 correlating with the increased chemotherapy dose. The Western blotting analysis confirmed that the total protein levels of HSP70 in the CM were significantly elevated in EPI-treated cells, without any corresponding change in the expression levels of HSP70 within the cancer cells themselves, indicating that the protein was secreted into the extracellular environment.
TAM Activation and Tumorigenic Effects: The interaction between chemotherapy-treated breast cancer cells and macrophages was assessed using co-culture systems. Conditioned media from macrophages co-cultured with EPI-treated MDA-MB-231 and MDA-MB-453 cells (referred to as THP-1/BC-EPI CM) significantly enhanced the pro-tumorigenic activity of macrophages.
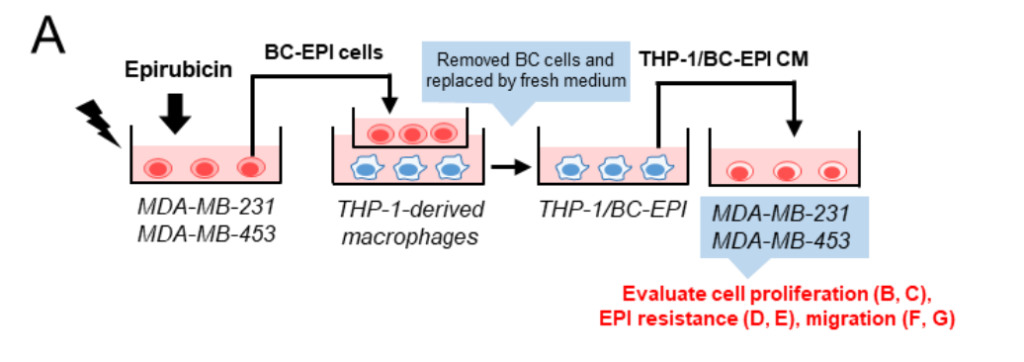
Specifically, TAMs exposed to the THP-1/BC-EPI CM exhibited a significant increase in their ability to promote cancer cell proliferation, survival in the presence of EPI, and migration compared to those exposed to CM from untreated cells.

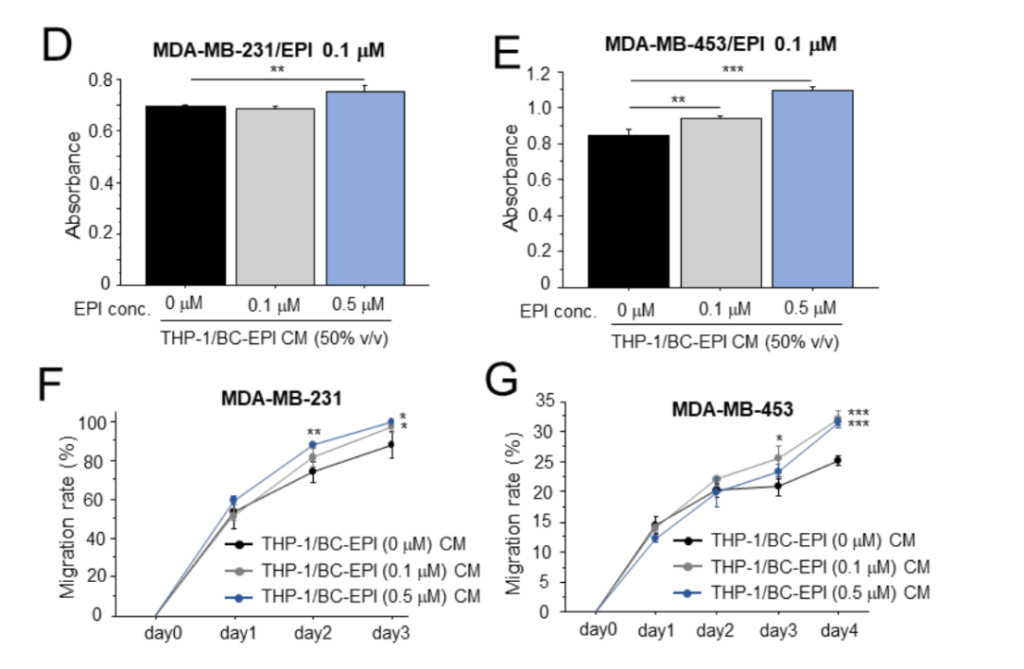
Cell Proliferation: A cell viability assay (using the Cell Counting Kit-8) showed that the CM from EPI-treated breast cancer cells (THP-1/BC-EPI CM) promoted a significant increase in the proliferation of MDA-MB-231 and MDA-MB-453 cells.
Chemoresistance: The chemoresistance assay revealed that macrophages exposed to THP-1/BC-EPI CM significantly enhanced the survival of breast cancer cells when exposed to EPI. The survival rate of MDA-MB-231 cells in the presence of EPI was higher with THP-1/BC-EPI CM compared to the control, indicating that the macrophages, under the influence of chemotherapy-induced HSP70, helped the cancer cells resist the effects of the drug.
Cell Migration: Migration assays, assessed through a wound-healing assay, showed that macrophages treated with the conditioned medium from chemotherapy-exposed breast cancer cells also increased cancer cell migration.
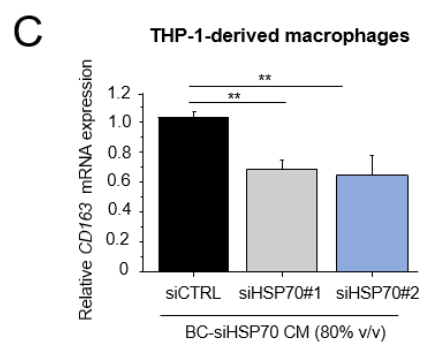
HSP70 and TGF-β Signaling in TAMs: The research also demonstrated a critical role for TGF-β signaling in mediating the pro-tumorigenic effects of macrophages. Using RNA interference (RNAi) to knockdown HSP70 in the breast cancer cells, the team observed a marked reduction in TGF-β expression in the macrophages when treated with CM from HSP70-silenced MDA-MB-231 and MDA-MB-453 cells. This suggests that extracellular HSP70 not only enhances the pro-tumorigenic function of macrophages but does so, at least in part, by modulating TGF-β expression in the tumor cells, which subsequently alters macrophage polarization and activity.
Macrophage Marker Expression: The immunohistochemical analysis of macrophage markers revealed that macrophages exposed to chemotherapy-induced HSP70 were skewed towards the M2 phenotype, characterized by increased expression of CD163, a marker commonly associated with pro-tumorigenic macrophages. The expression of CD163 in macrophages treated with CM from EPI-treated breast cancer cells was significantly higher compared to controls. This shift towards the M2 phenotype further supports the hypothesis that chemotherapy-induced HSP70 promotes a macrophage environment conducive to tumor progression and chemoresistance.
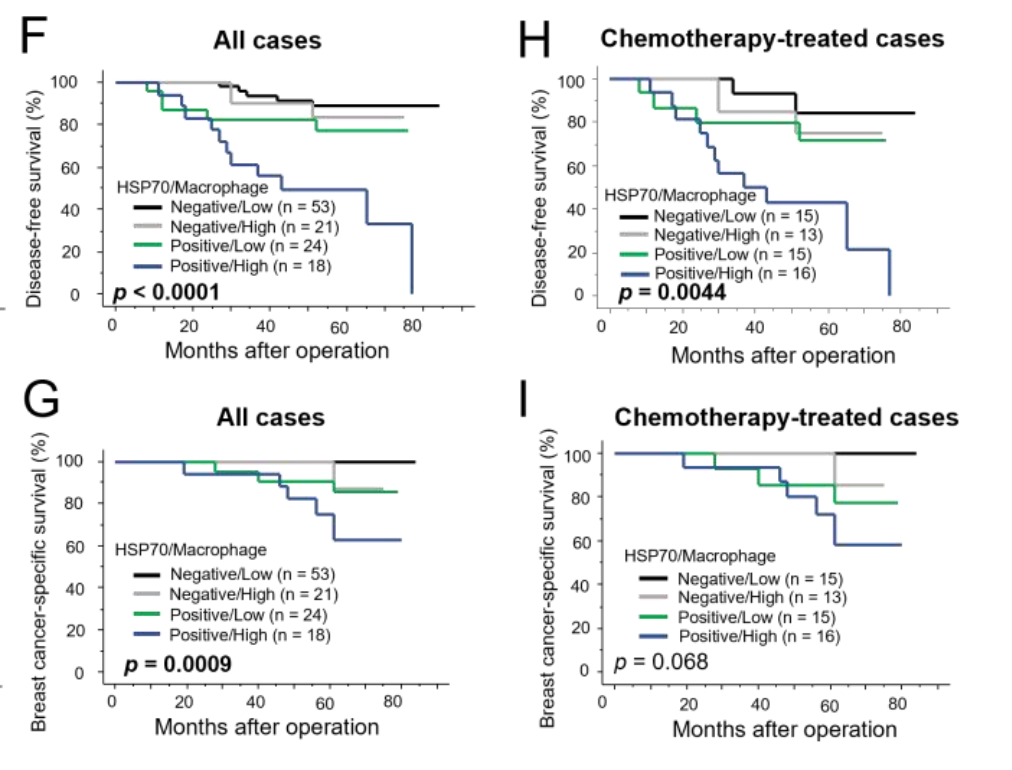
Clinical Relevance of HSP70 Expression: The clinical relevance of these findings was confirmed by analyzing 116 breast carcinoma specimens. The immunohistochemistry showed that the samples with high HSP70 expression had a concurrent increase in macrophage infiltration, and these samples were associated with poorer prognosis in terms of disease-free survival (DFS) and breast cancer-specific survival (BCSS). This correlation underscores the potential of HSP70 as a biomarker of chemoresistance and poor prognosis in breast cancer.
Conclusion of Results: In conclusion, the study provides strong evidence that chemotherapy-induced extracellular HSP70 plays a pivotal role in enhancing the pro-tumorigenic activity of TAMs through TGF-β signaling. The data suggests that targeting HSP70 or its interaction with TAMs could represent a promising therapeutic strategy to overcome chemotherapy resistance in breast cancer.
Conclusion: Targeting the HSP70-TAM Axis to Overcome Chemoresistance
The study concludes that extracellular HSP70, released following chemotherapy, significantly enhances the pro-tumorigenic effects of TAMs, either directly or indirectly, through TGF-β regulation. These findings suggest that HSP70 plays a crucial role in mediating chemoresistance in breast cancer by modifying macrophage behavior, which in turn promotes tumor progression.
The implications of this research are far-reaching, as targeting HSP70 or its interaction with TAMs could provide a novel therapeutic strategy to improve chemotherapy efficacy in breast cancer patients. However, the study’s reliance on in vitro models and the need for further validation in vivo highlight the limitations of the current approach. Future research should explore alternative macrophage-targeting therapies, as well as investigate the potential for HSP70 inhibitors to be used as adjuncts to chemotherapy.
By shedding light on the complex relationship between HSP70 and TAMs, this study opens the door to new strategies that could ultimately lead to better outcomes for breast cancer patients, particularly those with chemotherapy-resistant tumors.
Reference:
Yamaguchi-Tanaka, Mio, et al. “The pro-tumorigenic role of chemotherapy-induced extracellular hsp70 from breast cancer cells via intratumoral macrophages.” Cancers 15.6 (2023): 1903.
