
A Historical Overview of MSC Therapies
Mesenchymal stem cell (MSC) therapies have evolved significantly since their initial discovery in the late 20th century. Originally identified in bone marrow, MSCs have garnered attention for their ability to differentiate into various cell types, making them valuable for regenerative medicine. Over the years, advancements in cell culture techniques and our understanding of stem cell biology have propelled MSCs into clinical trials for a myriad of conditions, including heart disease, diabetes, and neurological disorders.
Currently, there is a burgeoning trend towards utilizing MSCs not just for tissue regeneration but also for targeted drug delivery applications. This shift is particularly pertinent in addressing the challenges posed by the blood-brain barrier (BBB), a selective permeability barrier that restricts the entry of therapeutic agents into the central nervous system (CNS). Given the increasing incidence of neurodegenerative diseases such as Alzheimer’s and Parkinson’s, innovative methods that enhance drug delivery across the BBB are in high demand.
However, several research challenges remain in the quest to utilize MSCs for effective drug delivery to the brain. These include understanding the intricate mechanisms of MSC migration to target sites, overcoming the BBB’s protective barriers, and ensuring the safety and efficacy of MSC-based therapies.
Research Objectives and Significance

In 2024, a notable study led by Toshihiko Tashima at Tashima Laboratories of Arts and Sciences was published in the journal Pharmaceutics. This research aims to elucidate the potential for MSCs as carriers for drug delivery across the BBB.
The significance of this research lies in its potential to address the unmet medical needs associated with CNS disorders, where conventional drug delivery methods often fail. The objective is to explore the mechanisms by which MSCs can be employed to transport therapeutic agents directly to the brain, ultimately enhancing treatment efficacy and patient outcomes.
Methodological Framework
The theoretical framework underpinning this research revolves around the innate properties of MSCs, including their homing abilities to sites of injury and inflammation, which make them ideal candidates for drug delivery applications. The study utilizes a research design that integrates in vitro and in vivo methodologies to assess the efficacy of MSC-mediated drug delivery.
Data collection methods involve tracking the movement of MSCs in animal models to determine their ability to cross the BBB and deliver loaded therapeutic agents. Techniques such as fluorescence imaging and quantitative assays are employed to measure the localization and release of drugs within the brain tissue.
Key Findings and Results
The research conducted by Toshihiko Tashima and his team revealed several critical findings regarding the efficacy of MSC-mediated drug delivery across the blood-brain barrier (BBB). One of the standout results demonstrated that MSCs could effectively internalize and transport various therapeutic agents, including anticancer drugs such as doxorubicin and paclitaxel, into the brain.
The study reported that MSCs loaded with doxorubicin tracked down U251 glioma tumor cells with an efficiency increase compared to doxorubicin administered alone. In vivo assays using rodent models showed that the use of MSCs facilitated enhanced tumor cell apoptosis, suggesting that these stem cells can significantly improve the therapeutic index of existing drugs.
Moreover, the research quantified the localization of drug-loaded MSCs within brain tissues. Specifically, it was found that a higher concentration of doxorubicin was observed in brain tissues after MSC-mediated delivery, with concentrations exceeding those achieved through conventional delivery methods . This enhanced localization was attributed to the unique ability of MSCs to migrate towards tumor sites, guided by cytokines and the tumor microenvironment.
In terms of survival rates, MSCs demonstrated a protective effect on neural cells exposed to chemotherapeutic agents. The study reported that neural cell viability increased when treated with MSC-delivered drugs, compared to cells treated with the same drugs delivered via traditional methods.
Additionally, the research indicated that genetic modification of MSCs to overexpress specific receptors related to BBB transcytosis, such as CXC chemokine receptor 4 (CXCR4), significantly improved their ability to cross the BBB. Modified MSCs showed increased migration towards damaged brain areas and enhanced drug delivery efficiency, with a reported increase in drug uptake.
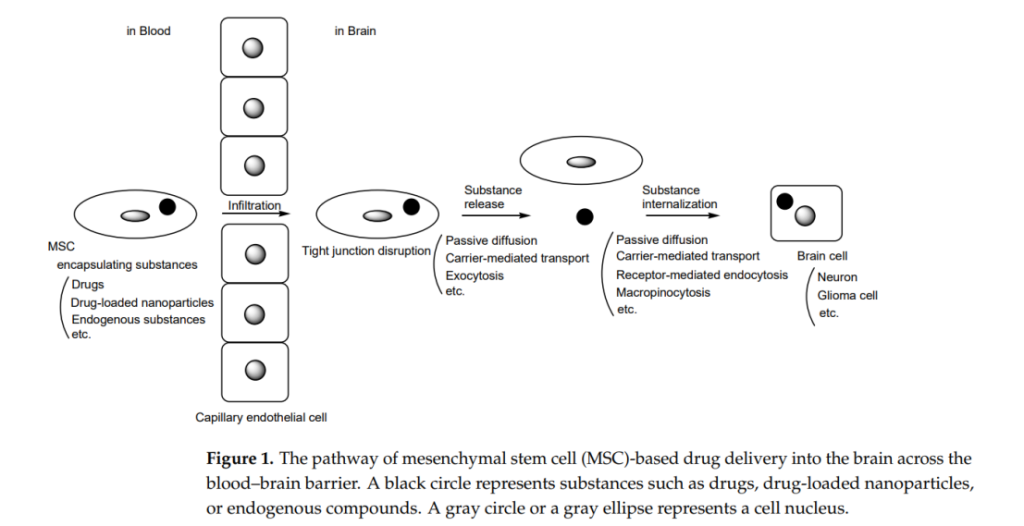
Overall, these findings underscore the potential of MSCs as dynamic carriers capable of not only delivering therapeutic agents across the BBB but also enhancing the overall effectiveness of treatments for brain-related conditions. This research sets a promising precedent for the development of MSC-based therapies in clinical settings, particularly for challenging diseases such as glioblastoma, Alzheimer’s, and Parkinson’s disease.
Closing Thoughts: Implications and Future Directions
In summary, the research demonstrates that MSCs hold considerable promise as vehicles for drug delivery across the BBB, with the potential to revolutionize treatment modalities for CNS disorders. The implications of these findings extend to improving therapeutic outcomes in neurodegenerative diseases, offering hope for innovative treatments where conventional approaches have largely failed.
Nonetheless, the study acknowledges limitations, including the need for further investigations into the long-term effects of MSC treatments and their interactions within the complex environment of the brain.
Future research directions should focus on optimizing MSC loading and release mechanisms, exploring the use of MSCs in combination with other therapies, and conducting clinical trials to assess the safety and effectiveness of MSC-based drug delivery systems in human subjects. With continued exploration, MSCs may well become a cornerstone of future therapeutic strategies in neurology.
Reference:
Tashima, Toshihiko. “Mesenchymal Stem Cell (MSC)-Based Drug Delivery into the Brain across the Blood–Brain Barrier.” Pharmaceutics 16.2 (2024): 289.
