A biomimetic, pH-responsive nanoplatform (CPCaNPs) for multimodal imaging and photothermal immunotherapy —— a potential leap forward in noninvasive cancer therapy.
Photothermal Therapy
Photothermal therapy (PTT) has emerged as a promising method for tumor ablation by converting light into thermal energy. However, traditional photothermal agents (PTAs) face significant challenges, including poor tumor targeting, brief circulation time, and potential toxicity. These issues limit the efficacy of PTT and pose risks of tumor recurrence and metastasis. Researchers have sought advanced solutions to address these shortcomings in cancer treatment.
A Vision for Precision Treatment
The research, led by Dr. Pan Li and Dr. Zhongqian Hu from Chongqing Medical University and Southeast University, respectively, aimed to develop CPCaNPs (CaCO3-PDA nanoparticles). These nanoplatforms, coated with cancer cell membranes and designed to be pH-responsive, enhance targeting accuracy while enabling imaging-guided PTT. The study, published in ACS Applied Materials & Interfaces in December 2023, explores how these particles address PTT’s existing limitations and synergize with immune checkpoint blockade (aPD-1) therapy to combat tumor recurrence.

Methodology
The CPCaNPs were synthesized via a dopamine-mediated biomineralization process. The coating with tumor cell membranes endowed the nanoparticles with homologous targeting abilities, enabling them to home in on tumor cells more effectively. Their pH sensitivity allowed them to decompose in the acidic tumor microenvironment, releasing CO2 bubbles to enhance ultrasound (US) imaging signals. The study also demonstrated how CPCaNPs combined photoacoustic (PA) imaging with photothermal efficacy for precise tumor ablation. Key experimental methods included:
- Evaluating CPCaNPs’ targeting and imaging functions.
- Analyzing their photothermal performance using near-infrared (NIR) lasers.
- Assessing the combined effects of CPCaNP-assisted PTT and aPD-1 therapy on tumor recurrence.
Results
- Enhanced Tumor Targeting:
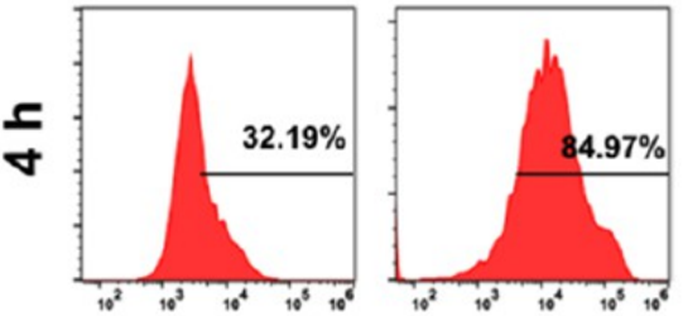
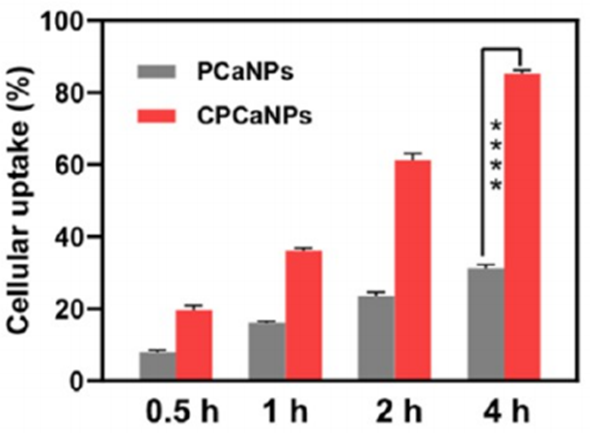
CPCaNPs showed 84.9% uptake by 4T1 cells after 4 hours, compared to 32.2% for uncoated PCaNPs, confirmed by confocal microscopy.
- Superior Imaging and Photothermal Efficiency:
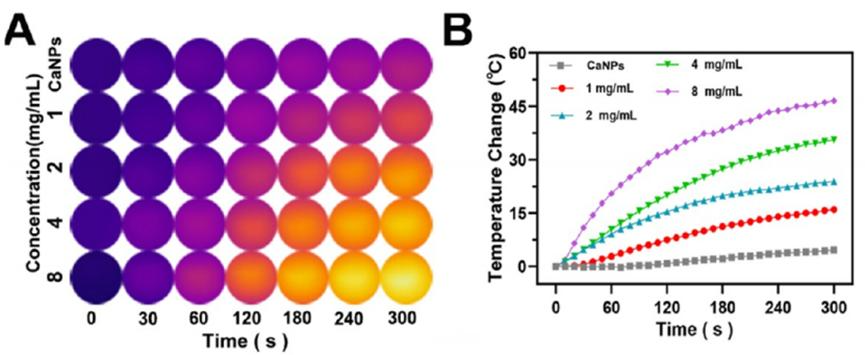
CPCaNPs increased the temperature from 19.6°C to 55.2°C at 4 mg/mL under 808 nm laser irradiation. Enhanced photoacoustic (PA) and ultrasound (US) signals were observed, with the highest CEUS at pH 5.5.
- Robust Anti-Tumor Response:
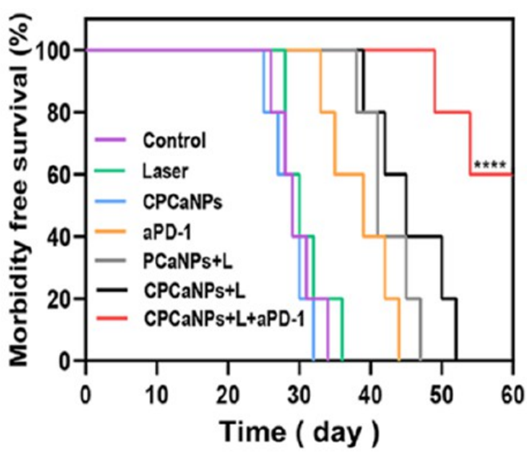
CPCaNPs at 4 mg/mL reduced cell viability to 21.8% after laser irradiation. In vivo, primary tumors were eliminated by day 16, and 60% of treated mice survived over 2 months.
- Immune Activation:
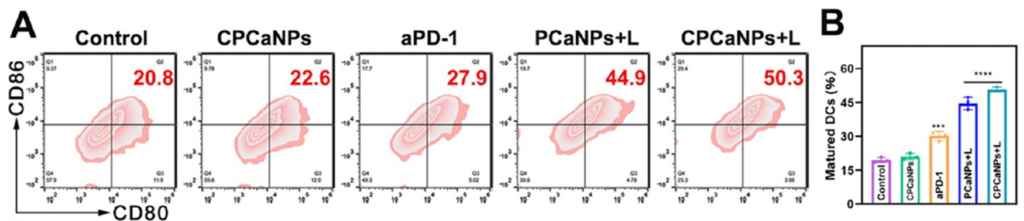
50.3% dendritic cell maturation and a 52.1% increase in cytotoxic T cells in distant tumors were observed. Cytokine levels for TNF-α, IFN-γ, and IL-12 were significantly higher (p < 0.0001).
Study Significance
This study introduces a biomimetic, pH-responsive CaCO3-PDA nanoparticle (CPCaNP) platform for tumor-targeted photothermal immunotherapy. Coated with cancer cell membranes, the nanoparticles exhibit enhanced targeting ability, allowing for precise tumor localization and minimizing the toxicity of photothermal agents. Upon exposure to the acidic tumor microenvironment, CPCaNPs disintegrate, generating CO2 bubbles to enhance ultrasound imaging, while the polydopamine (PDA) component facilitates tumor ablation through photoacoustic and thermal imaging guidance. This integrated approach improves tumor targeting and treatment accuracy, offering a more effective, noninvasive therapy.
In addition to photothermal effects, CPCaNPs, combined with immune checkpoint blockade (aPD-1), activate the immune system by promoting dendritic cell maturation and enhancing cytotoxic T cell infiltration. This combination effectively inhibits tumor recurrence and metastasis, showing potential for treating aggressive cancers like triple-negative breast cancer. While the results are promising, further studies are needed to assess long-term toxicity, the impact on tumor progression, and efficacy across different models. Overall, the CPCaNP platform holds promise for precise, minimally invasive cancer treatments in clinical applications.
Reference:
Wan, Li, et al. “Biomimetic, pH-responsive nanoplatforms for cancer multimodal imaging and photothermal immunotherapy.” ACS applied materials & interfaces 15.1 (2022): 1784-1797.
