Pioneering the Fight Against Glioblastoma
Glioblastoma multiforme (GBM), the most aggressive brain malignancy, has long challenged medical science due to its poor prognosis and limited treatment efficacy. Historically, therapeutic approaches relied heavily on surgery followed by radiotherapy and chemotherapy. While temozolomide (TMZ) emerged as a key chemotherapeutic agent, its efficacy has been marred by poor stability, rapid elimination, and drug resistance. Recent trends have turned toward nanoparticle-based drug delivery systems, which offer promising avenues for targeted and efficient therapy. However, GBM’s heterogeneity and its characteristic blood-brain barrier remain formidable barriers, underscoring the need for innovative solutions.
Breakthrough Objectives: Advancing GBM Treatment with Nanotechnology

A team of researchers from renowned institutions such as Dalian Medical University and Nanjing University has presented groundbreaking work in the journal Frontiers in Pharmacology. The study, led by Hao Wu and Tianyi Zhang, introduces polydopamine (PDA)-based nanoparticles conjugated with Peptide-1 (Pep-1) and loaded with TMZ. This novel approach aims to synergize chemotherapy and photothermal therapy (PTT), enhancing therapeutic precision and minimizing side effects. Their primary goal is to overcome GBM’s intrinsic challenges by developing a biocompatible, targeted nano-delivery system capable of penetrating the blood-brain barrier.
The Innovative Approach: Designing Smart Nanocarriers
Polydopamine nanoparticles were at the core of this study, celebrated for their strong adhesion, photothermal properties, and biocompatibility. The researchers employed a theoretical framework centered on the pH-responsive degradation properties of PDA and its ability to encapsulate TMZ effectively.
The research design included the synthesis of PDA-TMZ nanoparticles via dynamic Schiff base reactions, followed by the conjugation of Pep-1 for enhanced targeting. The nanoparticles underwent rigorous characterization using techniques like Fourier-transform infrared spectroscopy (FT-IR) and scanning electron microscopy (SEM). Data collection encompassed stability tests, cellular uptake analysis in glioma cells (U87), and in vivo evaluations using tumor-bearing mice to validate the dual-therapy efficacy.
Breakthrough Data on Polydopamine Nanoparticles
Unveiling the Nanoparticle Characteristics
The synthesized polydopamine nanoparticles (PDA NPs), PDA-TMZ NPs, Pep-1@PDA NPs, and Pep-1@PDA-TMZ NPs were uniform, spherical, and ranged in size from 122 nm to 140 nm. Detailed characterization using dynamic light scattering (DLS) and scanning electron microscopy (SEM) confirmed smooth surfaces and excellent dispersity. The nanoparticles demonstrated remarkable stability in water and fetal bovine serum over seven days, ensuring reliability during in vivo applications. The zeta potential measurements indicated values between -22.4 mV and -34.6 mV, suggesting strong stability and low risk of aggregation in biological systems.
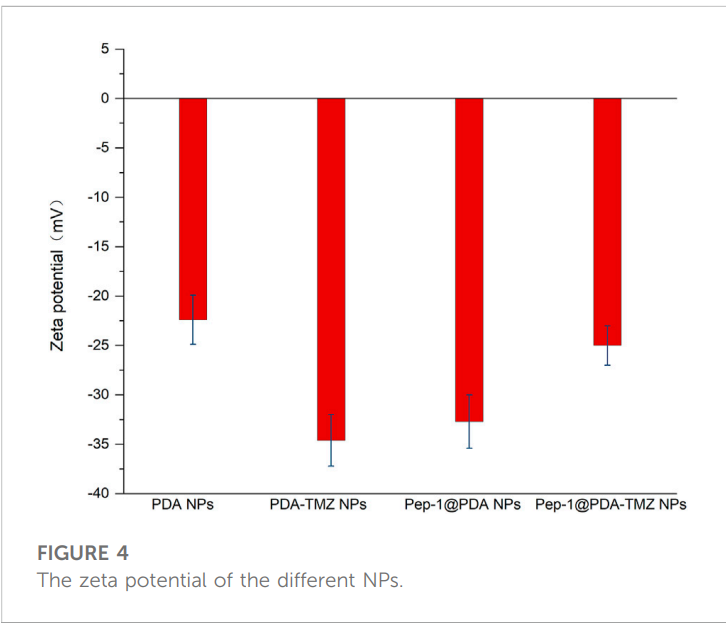
Enhancing Drug Delivery Efficiency
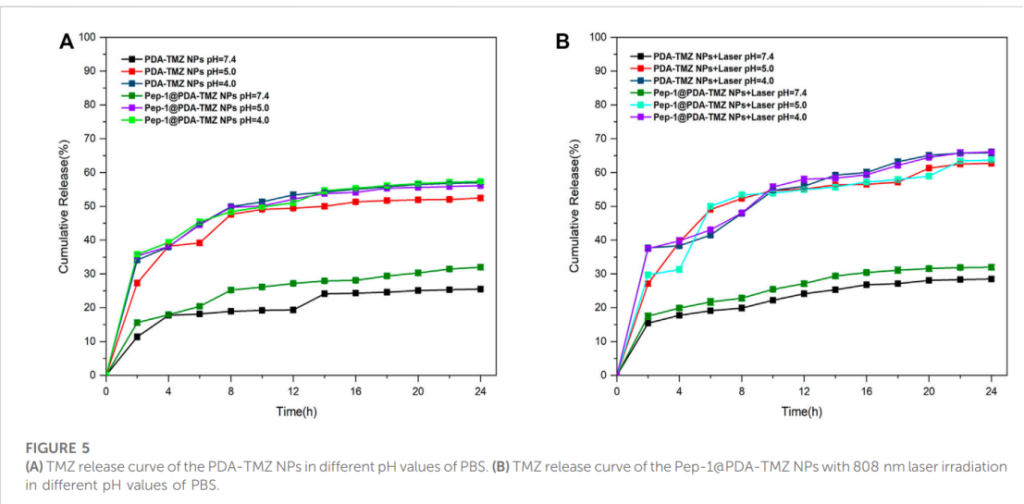
The encapsulation efficiency (EE) and loading rate (LR) of TMZ were noteworthy, reaching approximately 50% and 10.4%, respectively. Drug release studies in different pH environments revealed that the nanoparticles exhibited pH-responsive behavior. In acidic conditions mimicking the tumor microenvironment (pH 4.0 and 5.0), the cumulative release rates were 57.3% and 56.1%, respectively. Under laser irradiation at 808 nm, the release rates increased to 66.1%, demonstrating the synergy between photothermal therapy (PTT) and chemotherapy (CT).
Cytotoxicity and Cellular Uptake
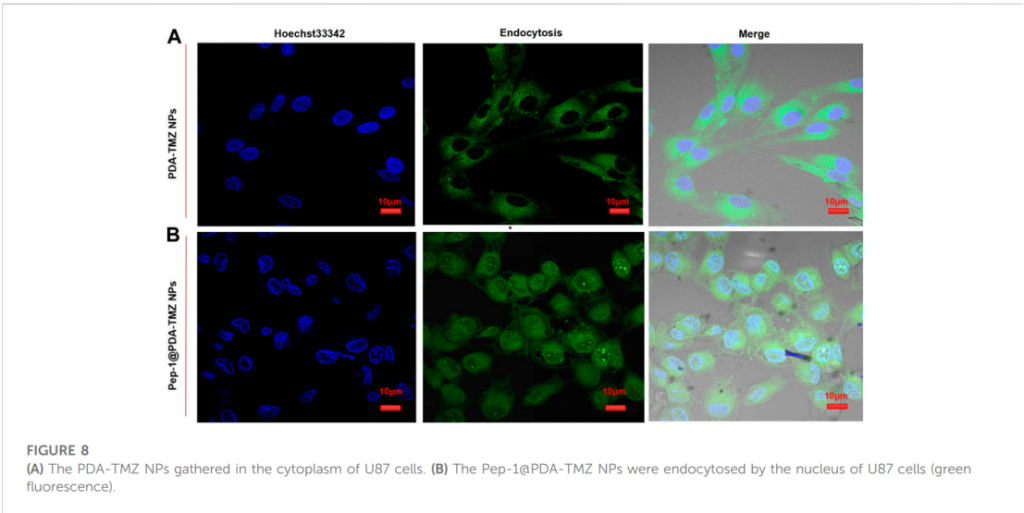
In vitro studies using U87 glioblastoma cells highlighted the therapeutic efficacy of the nanoparticles. Fluorescence imaging revealed that Pep-1 significantly enhanced nanoparticle penetration, enabling their accumulation in the nucleus, unlike PDA-TMZ NPs, which remained localized in the cytoplasm. This underscores the effectiveness of Pep-1 in facilitating targeted delivery through IL-13Rα2-mediated endocytosis.
In Vivo Tumor Suppression
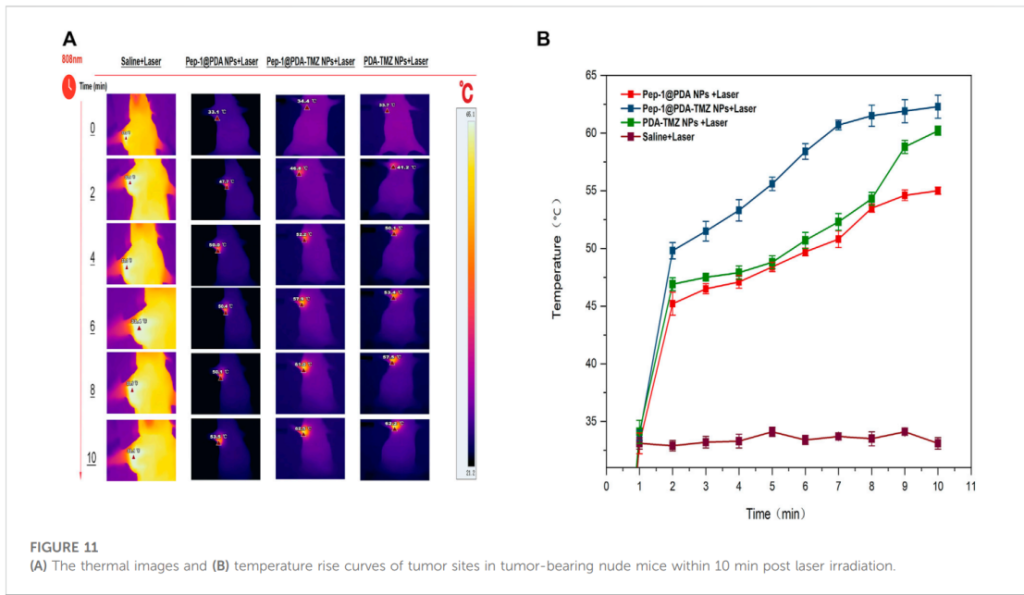
Tumor-bearing mice injected with Pep-1@PDA-TMZ NPs exhibited remarkable tumor growth inhibition. When combined with laser irradiation, tumor volumes were reduced by 77.13% compared to control groups. Thermal imaging confirmed that laser-treated tumors reached temperatures of approximately 60°C within 7 minutes, enabling effective ablation.
Importantly, there were no signs of significant toxicity in major organs, as confirmed by histological analysis. The treated mice maintained stable body weights, further indicating the biocompatibility of the nanoparticles.
These findings underscore the potential of Pep-1@PDA-TMZ NPs to serve as a transformative therapeutic platform, combining chemotherapy and photothermal therapy for glioblastoma with enhanced precision and reduced side effects.
Future Horizons: Optimizing Glioblastoma Care
This research highlights the transformative potential of PDA-based nanoparticles in glioblastoma therapy. By integrating chemotherapy with photothermal therapy, the study paves the way for more effective and less invasive treatments. However, limitations such as the need for further investigation into long-term safety and clinical applicability remain. Future research should explore broader tumor models and refine nanoparticle formulations to enhance targeting accuracy and therapeutic outcomes.
This groundbreaking work reaffirms the promise of nanotechnology in conquering GBM and inspires confidence in its eventual clinical translation.
Reference:
Wu, Hao, et al. “Polydopamine-based loaded temozolomide nanoparticles conjugated by peptide-1 for glioblastoma chemotherapy and photothermal therapy.” Frontiers in Pharmacology 14 (2023): 1081612.
