From Milk to Medicine: Tracing the Path of Exosome Innovation
Milk exosomes, naturally occurring nanocarriers, have a rich history rooted in their role in intercellular communication. Initially recognized for their nutritional and immunological benefits, exosomes derived from bovine milk have emerged as promising agents in biomedical applications. Their biocompatibility, stability under harsh conditions, and ability to encapsulate therapeutic molecules have propelled them to the forefront of drug delivery research. Today, efforts are focused on overcoming the key challenge of functionalizing these exosomes for tissue-specific drug targeting, a critical step toward clinical translation.
Optimizing Drug Delivery: A Collaborative Research Breakthrough

The research, conducted in 2023 by a team led by Dr. Sun Hwa Kim and Dr. Yoosoo Yang from the Korea Institute of Science and Technology, was published in Biomaterials Research. Their study aimed to refine a post-insertion modification technique for bovine milk exosomes, enhancing their potential as targeted drug carriers. By incorporating folate (FA) functional moieties onto exosome membranes, the team sought to create a platform capable of selective delivery to tumor cells with folate receptor (FR) expression.
Engineering Precision: The Science Behind Functional Milk Exosomes
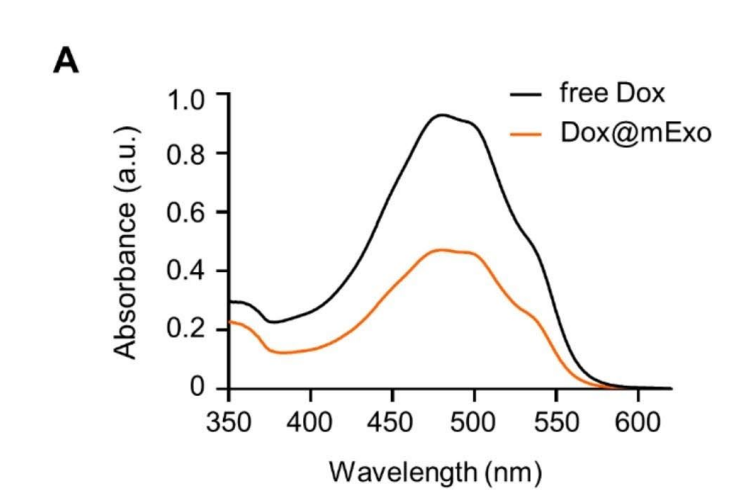
Milk-derived exosomes are a natural fit for drug delivery due to their stability and ability to shield therapeutic agents. This study employed a theoretical framework emphasizing hydrophobic insertion as a post-isolation modification strategy. FA-conjugated phosphatidylethanolamine (PE) lipids were integrated into the exosome membranes using a carefully optimized process. Validation of this functionalization was conducted through advanced techniques, including fluorescence imaging and spectrophotometric analysis, to confirm the successful incorporation of FA and evaluate drug-loading efficiency.
Precision in Action: Key Findings from the Study
The study yielded compelling evidence supporting the efficacy of functionalized milk exosomes in targeted drug delivery. FA-functionalized milk exosomes (mExo-FA) demonstrated enhanced cellular uptake via folate receptor (FR)-mediated endocytosis in FR-positive cancer cells such as HCT116, compared to non-functionalized exosomes. Confocal fluorescence imaging revealed significant intracellular localization of FA-modified exosomes in these cells, confirming their specificity.
The loading efficiency of the chemotherapeutic agent doxorubicin (Dox) into mExo-FA was approximately 30–35%.
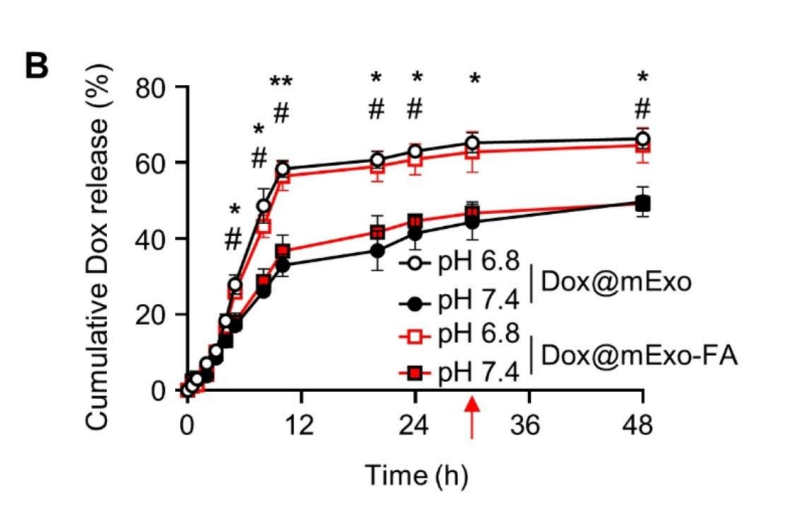
Moreover, the release profile showed a sustained and pH-sensitive Dox release, with 64.6% of the drug released under mildly acidic conditions (pH 6.8), mimicking the tumor microenvironment, compared to 50.7% under neutral pH. This pH-dependent behavior supports the preferential drug release at tumor sites, minimizing systemic exposure and reducing potential side effects.
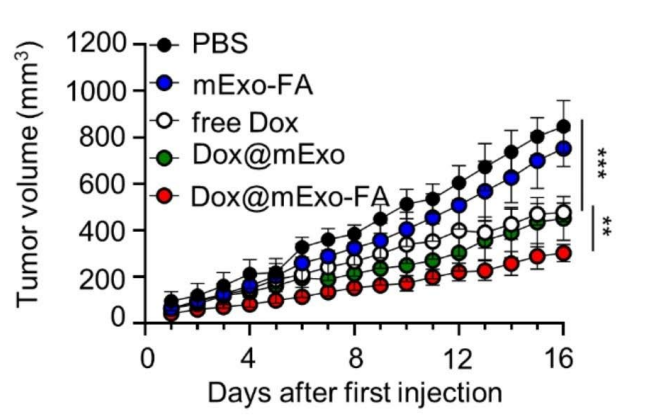
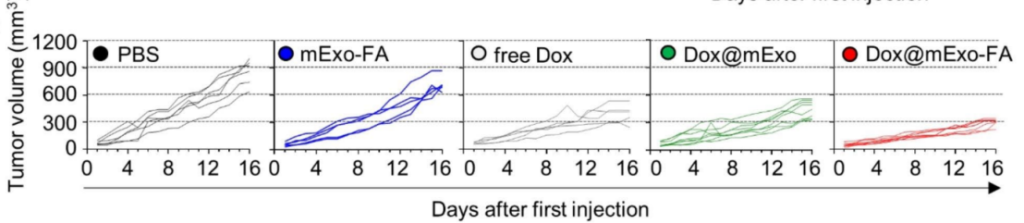
In vivo experiments further validated the tumor-targeting capabilities of mExo-FA. In HCT116 tumor-bearing mice, the exosomes exhibited significantly higher tumor accumulation compared to non-functionalized exosomes, as visualized through fluorescence imaging. This targeted delivery translated to superior therapeutic outcomes; Dox-loaded mExo-FA (Dox@mExo-FA) substantially inhibited tumor growth compared to controls, including free Dox and non-functionalized exosomes. Tumor volumes in mice treated with Dox@mExo-FA were markedly smaller, with a final average of 302.7 mm³, compared to 477.6 mm³ in the free Dox group.
Histological analysis via TUNEL assays revealed enhanced apoptotic cell death in the Dox@mExo-FA group, further corroborating the therapeutic advantage.
The systemic safety profile of Dox@mExo-FA was noteworthy. Unlike free Dox, which caused significant weight loss (29.5% reduction) and splenic atrophy in treated mice, Dox@mExo-FA preserved body weight and organ integrity, as confirmed by histological analysis.
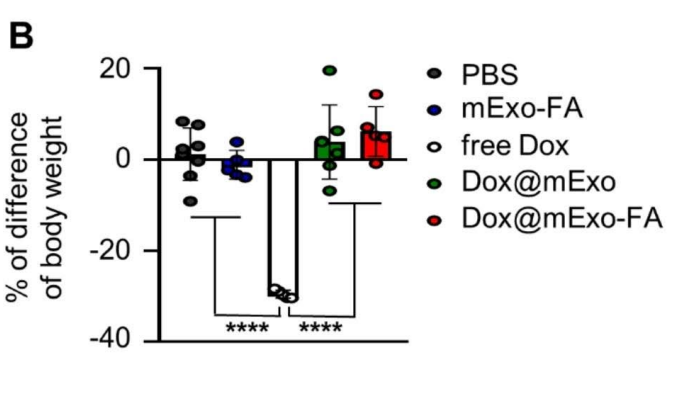
Additionally, liver enzyme levels (AST and ALT) and cardiac tissue analysis indicated reduced toxicity in the exosome-delivered group, addressing a critical limitation of conventional Dox administration.
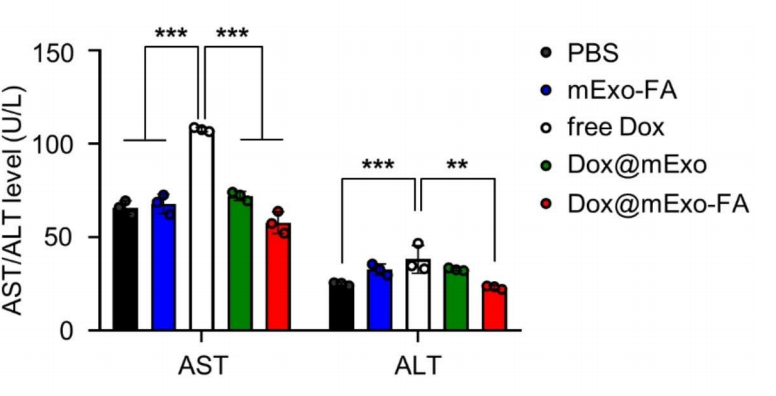
These findings demonstrate that the FA-functionalized exosome platform not only enhances drug delivery efficiency but also mitigates systemic toxicity, paving the way for safer and more effective cancer therapies.
Shaping the Future: Implications and Next Steps in Exosome Research
This research underscores the potential of milk-derived exosomes as versatile, biocompatible drug carriers capable of targeted delivery. The FA-functionalized exosomes not only enhanced therapeutic efficacy but also minimized systemic toxicity, marking a pivotal step in their application for cancer treatment. However, challenges remain, including addressing the extracellular matrix barriers in tumor microenvironments and exploring multi-functional modifications for broader applications. Future research is poised to refine these strategies and expand the clinical utility of milk exosome-based platforms.
Conclusion
This study highlights a groundbreaking approach to leveraging milk exosomes for targeted drug delivery, showcasing their ability to enhance therapeutic precision and safety. With continued innovation, milk exosomes are set to revolutionize the field of nanomedicine, offering a cost-effective and scalable solution for addressing complex therapeutic challenges. The promise they hold could pave the way for transformative advancements in cancer therapy and beyond.
Reference:
Jang, Hochung, et al. “Post-insertion technique to introduce targeting moieties in milk exosomes for targeted drug delivery.” Biomaterials Research 27.1 (2023): 124.
