A study reveals that low-dose inorganic nanoparticles can promote liver health by combating fatty liver disease, offering a potential new approach to metabolic disorder treatments.
Background — Targeting Fatty Liver with a Novel Approach
Non-alcoholic fatty liver disease (NAFLD) has emerged as a significant global health issue, affecting over 25% of the world’s population. Characterized by abnormal fat accumulation in the liver, NAFLD increases the risk of liver damage, diabetes, and cardiovascular diseases. Current treatment options are limited, relying on lifestyle changes and lacking effective pharmacological interventions.
The study, conducted by researchers from Zhejiang University and published in Nature Communications (2023), explores an unconventional solution—low-dose inorganic nanoparticles. These particles, often used in biomedicine, exhibit unique properties that may help overcome the limitations of existing therapies by targeting liver cells directly.
Research Objectives — Exploring a Nanoparticle-Led Solution
The research aimed to evaluate the effects of orally administered inorganic nanoparticles, including titanium dioxide (TiO₂), gold (Au), and sodium yttrium fluoride (NaYF₄), on hepatic lipid metabolism. Specifically, the team investigated how these nanoparticles modulate reactive oxygen species (ROS) levels in the liver and activate lipid-degrading enzymes. Led by Zhejiang University’s multidisciplinary team, this study represents a significant stride in nanomedicine and metabolic research.
Methodology — How Nanoparticles Were Tested in Action
The researchers administered low doses of TiO₂, Au, and NaYF₄ nanoparticles (less than 1 mg/kg/day) to both wild-type and obese mouse models. Using advanced imaging and molecular analysis, they tracked the nanoparticles’ journey from gastrointestinal absorption to their accumulation in liver cells.
The nanoparticles induced a modest increase in ROS, which activated the expression of carboxylesterase 2h (Ces2h), a liver enzyme responsible for breaking down fat molecules. Crucially, the nanoparticles did not cause significant toxicity or damage to the liver or other organs.
Results — Proof of Concept: Tackling Fatty Liver
The findings of the study provide compelling evidence for the therapeutic potential of nanoparticles in addressing fatty liver disease:
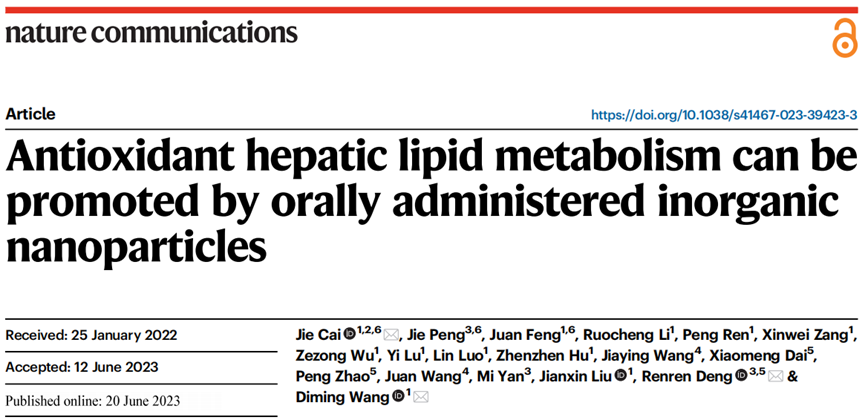
1. Reduction in Liver Fat Levels: Low-dose nanoparticle treatment reduced hepatic triglyceride and cholesterol levels in mice by up to 47%.
The reduction spanned multiple lipid categories, including free cholesterol and cholesterol esters. Plasma β-hydroxybutyrate, a marker of fatty acid oxidation, also increased significantly, from approximately 400 to 550 μM, highlighting improved lipid metabolism.
2. Improved Liver Function and Structure: Histological analyses revealed a marked decrease in fat accumulation in liver tissue, evidenced by a reduced Oil Red O staining area in treated groups. Liver steatosis severity diminished considerably compared to untreated controls, supporting the nanoparticles’ efficacy in mitigating lipid overload.
3. Selective Targeting of Hepatocytes: Nanoparticles selectively targeted hepatocytes, as demonstrated by dynamic imaging studies. After 24 hours of administration, relative fluorescence intensity within hepatocytes rose substantially, indicating preferential accumulation of nanoparticles in these cells.
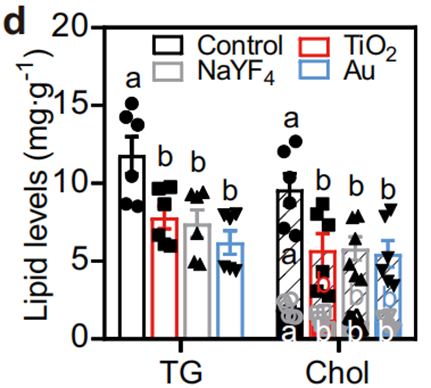
4. Therapeutic Impact on Obese Models: In genetically obese db/db mice, nanoparticle administration over 92 days resulted in a 20% body weight reduction and a 31.1–43.6% decrease in hepatic lipid content.
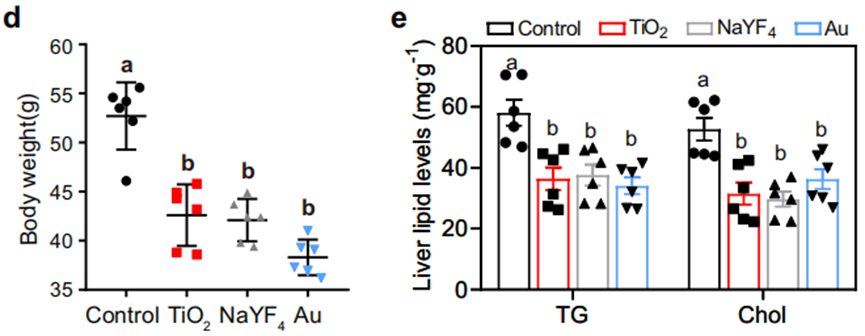
Similar benefits were observed in high-fat diet-induced obese mice, with liver enzyme levels (ALT and AST) returning to near-normal ranges, suggesting alleviation of liver damage.
5. Biochemical Insights: Transcriptomic analysis showed elevated Ces2h expression in nanoparticle-treated mice. This gene, pivotal for lipid hydrolysis, was upregulated specifically in response to nanoparticles, aligning with increased fatty acid oxidation-related gene expression, such as Cpt1b and Acox1.
These results collectively underscore the nanoparticles’ ability to not only reduce lipid accumulation but also enhance metabolic processes critical to liver health.
Significance of the Study
This research highlights a novel mechanism where low-dose nanoparticles act as catalysts for metabolic regulation in the liver. By inducing a controlled ROS response and upregulating Ces2h expression, these nanoparticles provide a safe and effective strategy to alleviate lipid metabolism disorders without causing systemic toxicity.
Reference:
Cai, Jie, et al. “Antioxidant hepatic lipid metabolism can be promoted by orally administered inorganic nanoparticles.” Nature Communications 14.1 (2023): 3643.
