A recent study published in the Journal of Drug Delivery Science and Technology reveals a novel nanocomposite material that exhibits synergistic antibacterial and antibiofilm activities.

Background: Challenges in Revolutionizing Antibacterial Treatments
The growing prevalence of bacterial infections and antibiotic resistance has posed significant challenges to traditional treatment approaches. Misuse of antibiotics has accelerated the emergence of resistant strains, while biofilm formation by bacteria further exacerbates the problem by creating protective barriers that limit the efficacy of drugs. Current therapeutic options are insufficient, prompting a surge of interest in innovative solutions. Among these, nanoparticles have emerged as a promising tool due to their high surface area, enhanced penetration abilities, and potent antimicrobial properties.
Research Aim: A Breakthrough Dual-Action Therapy
Researchers from Tehran Medical Sciences, Islamic Azad University, and other affiliated institutions developed a novel nanocomposite system aimed at enhancing antibacterial and antibiofilm effectiveness. This innovative approach combines Sultamicillin tosylate with silver and zinc oxide nanoparticles, encapsulated in a carboxymethyl chitosan (CMC)-coated niosome, to achieve improved drug stability and targeted delivery.
The study, led by a team including Shamim Ashkezari and Maryam Sadat Abtahi, was published online on March 31, 2023. Their work explores the synergistic potential of nanotechnology-based drug delivery systems to combat resistant bacterial infections.
Methodology: Engineering a Next-Generation Antimicrobial System
The research draws inspiration from advancements in nanotechnology and drug delivery systems. Using the thin-film hydration method, the team synthesized a nanocomposite that incorporates Sultamicillin tosylate alongside silver or zinc oxide nanoparticles into a niosome carrier. This structure was further coated with CMC, enhancing its stability, controlled release, and dual-action antibacterial mechanisms.
The optimized niosomes demonstrated key attributes such as a particle size of 139.60 nm, a polydispersity index (PDI) of 0.229, and an encapsulation efficiency of 73.45%. Stability testing confirmed the formulation’s robustness over 30 days, while drug release studies showed a sustained release of 65.84% over 48 hours.
Results: Promising Outcomes Against Resistant Bacteria
The nanocomposite demonstrated exceptional antibacterial and antibiofilm efficacy across multiple bacterial strains, including Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, and Klebsiella pneumoniae. The optimized CMC-coated formulations (e.g., ST-Nio-AgNPs@CMC and ST-Nio-ZnONPs@CMC) significantly outperformed uncoated counterparts in reducing biofilm formation and bacterial growth. Key data include:
- Biofilm reduction: The CMC-coated formulations reduced biofilm biomass by up to 75%, compared to free drugs, as confirmed by crystal violet assays.
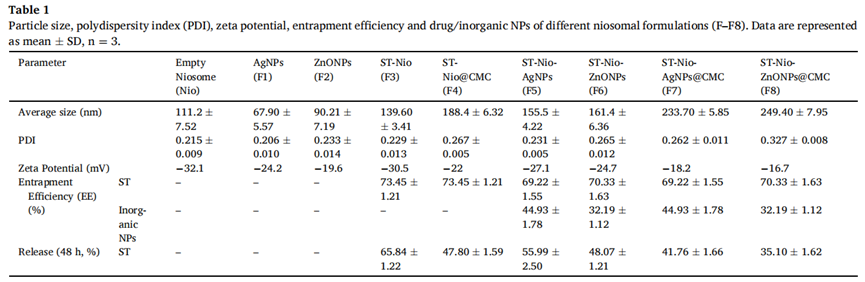
- Gene expression analysis: Real-time PCR showed significant downregulation of biofilm-associated genes, such as icaA (in S. aureus), MrkA (in K. pneumoniae), FimH (in E. coli), and arr (in P. aeruginosa), with fold changes greater than twofold compared to untreated controls (p ≤ 0.001).
- Antibacterial activity: The minimum inhibitory concentration (MIC) of the CMC-coated formulations was reduced by up to fourfold compared to free Sultamicillin, highlighting enhanced antibacterial potency. For example, the MIC for P. aeruginosa dropped to 0.78 μg/mL with ST-Nio-AgNPs@CMC.
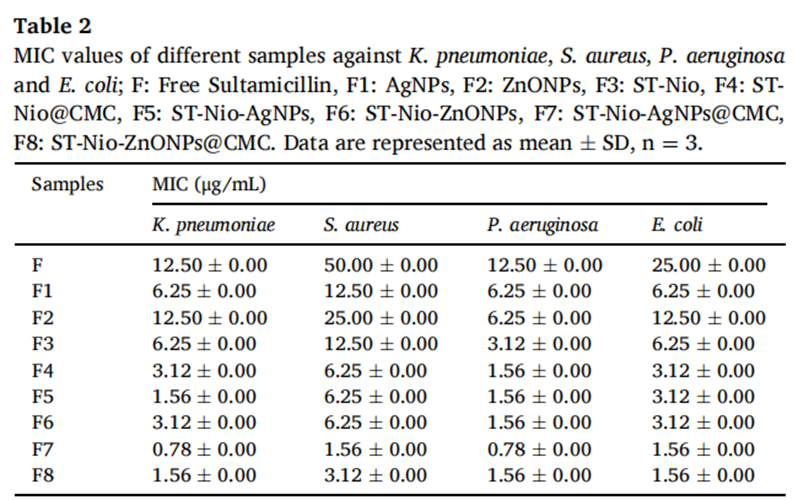
- Drug release: The formulations achieved sustained drug release, with cumulative release rates for ST-Nio-AgNPs@CMC and ST-Nio-ZnONPs@CMC measured at 44.06% and 38.88%, respectively, over 72 hours.

Conclusion: Advancing Biomedical Applications
This study demonstrates the successful development of a novel dual-action nanocomposite system, co-loading Sultamicillin tosylate with silver or zinc oxide nanoparticles into a CMC-coated niosome. The formulation exhibited significant improvements in antibacterial and antibiofilm activities against both gram-positive and gram-negative bacteria. These enhancements are attributed to the synergistic effects of Sultamicillin and inorganic nanoparticles, as well as the stability and controlled-release properties conferred by the CMC coating.
Reference:
Ashkezari, Shamim, et al. “Antibiotic and inorganic nanoparticles co-loaded into carboxymethyl chitosan-functionalized niosome: Synergistic enhanced antibacterial and anti-biofilm activities.” Journal of Drug Delivery Science and Technology 83 (2023): 104386.
