Introduction: A New Chapter in Antibacterial Innovation
Nanoparticles have long been heralded as a beacon of hope in combating drug-resistant bacterial infections. Among the myriad innovations, the advent of Janus nanoparticles marks a pivotal shift. Named after the two-faced Roman deity, these asymmetric structures leverage distinct properties for targeted antibacterial solutions. Historically, symmetrical nanoparticles have dominated the field but lacked precision in penetrating biofilms—a crucial challenge given that biofilms shield bacteria from conventional therapies.
In recent developments, Janus-structured nanoparticles, specifically those integrating dextran and bismuth selenide (BSe), have emerged as a game-changer. These particles not only disperse biofilms but also exhibit photothermal effects activated by near-infrared (NIR) light. However, the journey to this breakthrough was fraught with challenges, particularly the limited penetration and efficacy of existing photothermal nanoparticles in dense biofilm matrices. The research discussed here addresses these gaps, presenting a robust solution to eradicate drug-resistant biofilms effectively.
The Objective: Engineering a Revolutionary Antibacterial Tool

This groundbreaking study, conducted by Zhiwen Liu, Kangli Guo, Liemei Yan, and their colleagues from the Beijing University of Chemical Technology, was published in Nature Communications. The team’s mission was to develop Janus-structured Dex-BSe nanoparticles capable of targeting biofilms with unprecedented precision. Combining dextran’s biofilm-affinity with BSe’s photothermal properties, these nanoparticles aim to revolutionize biofilm-targeted antibacterial therapy while exploring their dual functionality—biofilm dispersion and photothermal eradication.
Methodology: Crafting the Janus Marvel
The synthesis of Dex-BSe nanoparticles involved a nonsolvent-aided counterion complexation process. Using ethanol as the nonsolvent, the team achieved a high-yield production of nanoparticles characterized by their dual-domain structure: a dextran-rich surface for biofilm targeting and a BSe-rich edge for photothermal activity.
The research followed a meticulous design, encompassing both in vitro and in vivo experiments. In vitro studies evaluated the nanoparticles’ penetration and dispersion capabilities in Staphylococcus aureus biofilms, while in vivo tests used mouse models of wound infections and abscesses to validate photothermal efficacy. Data collection methods included advanced tools such as confocal microscopy for biofilm imaging, RNA sequencing for molecular analysis, and thermal performance assessments to measure photothermal efficiency.
Results: A Dual-Action Triumph in Antibacterial Therapy
The Janus Dex-BSe nanoparticles demonstrated exceptional performance in overcoming biofilm-related challenges, supported by detailed in vitro and in vivo analyses.
Enhanced Biofilm Penetration
Confocal laser scanning microscopy (CLSM) imaging revealed that Janus Dex-BSe nanoparticles rapidly penetrated biofilms within 30 minutes. The nanoparticles showed significant accumulation throughout the biofilm matrix, with red fluorescence indicating their deep infiltration compared to the limited penetration of CS-BSe nanoparticles under identical conditions.
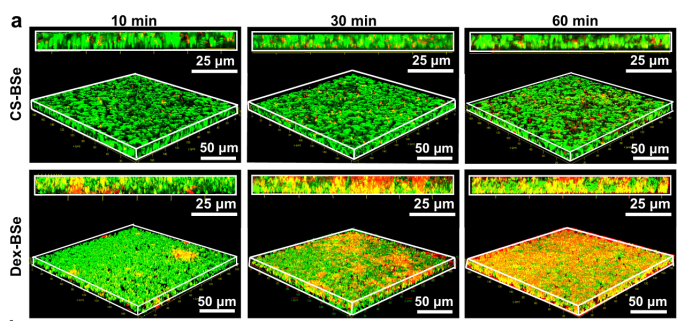
Effective Biofilm Dispersion
Over an extended incubation period of 6 hours, Dex-BSe nanoparticles led to a marked reduction in biofilm integrity. Quantitative analyses showed a significant decrease in bacterial biomass, as evidenced by crystal violet staining and dry weight assessments.
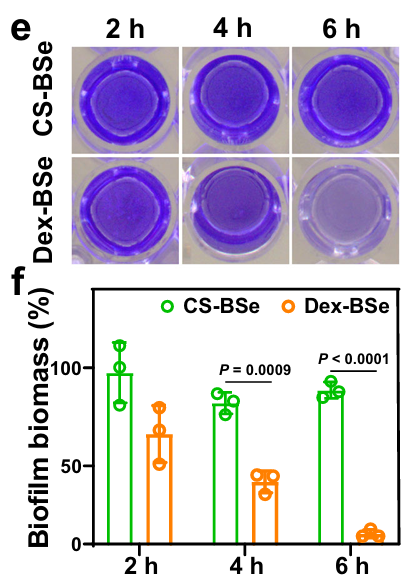
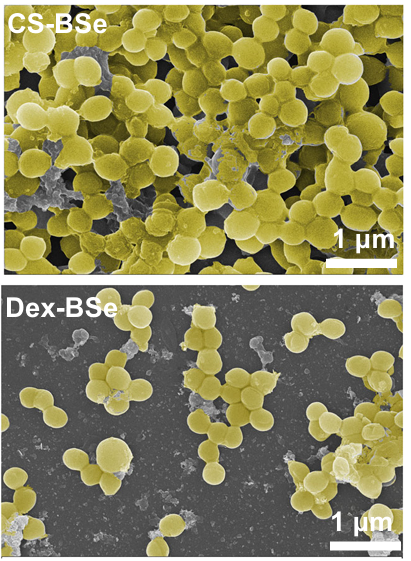
The bacterial colony-forming units (CFUs) were reduced by over 60%, underscoring the nanoparticles’ superior biofilm dispersion capabilities. These findings were corroborated by scanning electron microscopy (SEM), which illustrated fewer residual bacterial cells compared to untreated biofilms.
Superior Photothermal Performance
The nanoparticles exhibited a photothermal conversion efficiency of 31.5%, as determined from temperature elevation experiments under near-infrared (NIR) light irradiation. This efficiency enabled the nanoparticles to reach sufficient temperatures for effective bacterial eradication. Furthermore, the active motion induced by NIR-triggered heating enhanced the photothermal effects, making the nanoparticles highly effective in biofilm-related antibacterial applications.
These results collectively highlight the advanced capabilities of Janus Dex-BSe nanoparticles in addressing biofilm-associated infections through their dual functionality—biofilm targeting and photothermal therapy.
Conclusion: Charting the Future of Antibacterial Therapies
This study underscores the transformative potential of Janus Dex-BSe nanoparticles in overcoming biofilm-associated infections. Their dual functionality—targeting biofilm dispersion and delivering photothermal therapy—sets a new standard in antibacterial treatments. Beyond immediate applications, the research holds broader implications for wound care, medical device coatings, and infection control in healthcare settings.
While the nanoparticles demonstrated promising results, limitations such as variability in biofilm composition and scalability of synthesis were noted. Future research is recommended to optimize the formulation for targeting Gram-negative bacteria and exploring synergistic combinations with other therapeutic agents.
As the fight against drug-resistant infections intensifies, innovations like the Janus Dex-BSe nanoparticles offer a beacon of hope, paving the way for safer, more effective antibacterial strategies.
Reference:
Liu, Zhiwen, et al. “Janus nanoparticles targeting extracellular polymeric substance achieve flexible elimination of drug-resistant biofilms.” Nature Communications 14.1 (2023): 5132.
