A bacteria-responsive hydrogel with self-activating antibacterial properties offers a promising solution for accelerating the healing of infected wounds.

Background
Infected wounds, particularly those associated with chronic bacterial infections, present significant challenges to global healthcare. These wounds often experience prolonged inflammation, impaired angiogenesis, and the formation of biofilms that hinder healing. Conventional treatments, including antibiotics and dressing materials, face limitations such as poor bioavailability, inefficacy against drug-resistant bacteria, and inability to address the complex microenvironment of infected wounds. These challenges highlight an urgent need for advanced therapeutic strategies that not only target bacteria but also promote tissue regeneration.
Research Objectives and Innovation
To address these challenges, a team of researchers from Xi’an Jiaotong University and Tangdu Hospital developed an intelligent hydrogel dressing capable of self-activating antibacterial and regenerative functions. The research, led by Professor Baolin Guo and colleagues, focused on a composite hydrogel designed to respond to the dynamic microenvironment of infected wounds. Published in January 2024, the study introduces a novel wound dressing platform that combines bacteria-responsive, pH-sensitive micelles and nanozyme technology. This innovative approach aims to eradicate biofilms, relieve oxidative stress, and accelerate angiogenesis, offering an integrated solution to wound healing.

Research Methods
The hydrogel, named CAOP/M/PL, incorporates dynamic Schiff base and phenylboronate bonds for self-healing properties. It leverages stimuli-responsive micelles loaded with lactate oxidase (Lox) and a composite nanozyme (MSCO) that reacts to bacterial metabolites. When exposed to acidic wound environments, the hydrogel releases antibacterial agents, including hydrogen peroxide (H2O2) and reactive oxygen species (ROS), while simultaneously generating nitric oxide (NO) to weaken bacterial defenses. This multi-faceted mechanism allows the hydrogel to adapt its antibacterial and regenerative effects throughout the healing process.
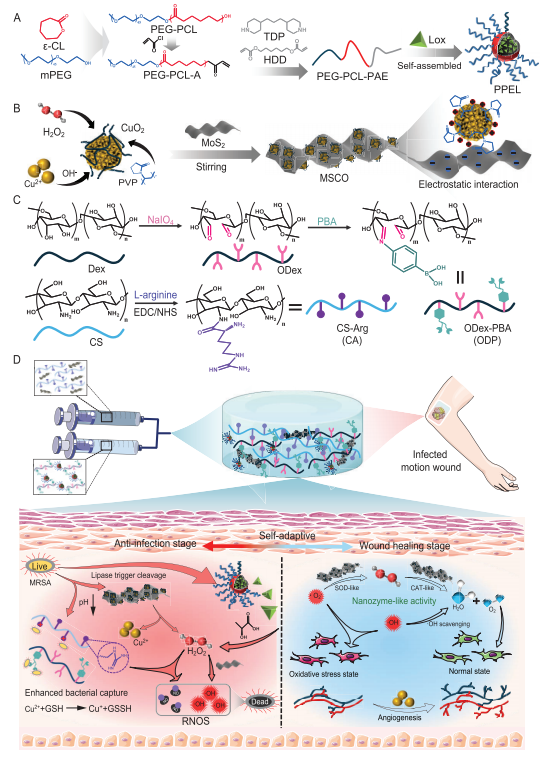
Results
The hydrogel demonstrated remarkable efficacy in both in vitro and in vivo experiments.
Antibacterial Efficiency: The hydrogel eliminated over 99% of Escherichia coli and methicillin-resistant Staphylococcus aureus (MRSA) at acidic pH levels. It disrupted biofilms, leaving less than 17% residue compared to over 70% in conventional treatments.
Regenerative Performance: The hydrogel enhanced angiogenesis and tissue regeneration in mouse wound models. By the 15th day of treatment, wounds treated with CAOP/M/PL were almost fully closed, while control wounds remained significantly larger. The hydrogel also promoted collagen deposition and hair follicle formation, indicating robust tissue repair.
Oxidative Stress Relief: The nanozyme activity within the hydrogel efficiently scavenged excess ROS, reducing oxidative stress that can impede healing. This dual action of combating infection and promoting a regenerative microenvironment underscores the hydrogel’s versatility.
Angiogenesis and Cell Proliferation: The hydrogel significantly increased human umbilical vein endothelial cell (HUVEC) proliferation and tubule formation. Compared to controls, the CAOP/M/PL hydrogel group achieved a 276% increase in hair follicle formation and the highest VEGF (vascular endothelial growth factor) expression, critical for new blood vessel growth.
These results highlight the hydrogel’s ability to not only fight infections but also restore damaged tissues effectively.
Conclusion
This study presents an innovative antibacterial hydrogel designed to address challenges in infected wound treatment by remodeling the regeneration microenvironment. The CAOP/M/PL hydrogel demonstrates intelligent bacteria-responsive capabilities, including self-activated antibacterial properties and the ability to dynamically regulate oxidative stress and promote angiogenesis. The findings highlight its effectiveness in reducing bacterial infections, eradicating biofilms, and accelerating tissue regeneration.
The hydrogel’s self-healing properties, combined with its ability to meet the demands of motion wound environments, provide a promising method for designing advanced wound dressings. These results suggest that this hydrogel represents a significant step forward in the development of intelligent therapies for infected wounds.
Reference:
Yang, Yutong, et al. “Bacteria-responsive programmed self-activating antibacterial hydrogel to remodel regeneration microenvironment for infected wound healing.” National Science Review 11.4 (2024): nwae044.
