Background
Lyme disease, caused by the bacterium Borrelia burgdorferi, remains the most common vector-borne illness in the United States. With approximately 476,000 cases diagnosed annually, it poses a growing public health challenge. The disease is transmitted through the bite of infected Ixodes ticks and often begins with the appearance of erythema migrans, a red, bullseye-shaped rash. As the infection progresses, patients may experience flu-like symptoms, including fever, fatigue, and muscle aches.
If untreated or if treatment is delayed, Lyme disease can lead to severe complications, such as Lyme carditis, arthritis, and neurological issues, which can persist for years and significantly impair the quality of life. Unlike some other infectious diseases, no human vaccine for Lyme disease has been available since the withdrawal of LYMErix in 2002 due to concerns about side effects and low public acceptance. Treatment currently relies on antibiotics, such as doxycycline, which are effective in early stages but offer limited efficacy against late-stage complications and do not prevent reinfection with different Borrelia strains. This highlights an urgent need for a safe, effective, and long-lasting vaccine.
Research Aim & Objectives
In a groundbreaking effort, researchers from the University of Pennsylvania and Yale University, led by Dr. Norbert Pardi and Dr. Erol Fikrig, have developed a novel mRNA-based vaccine to combat Lyme disease. Their study, published in Molecular Therapy in 2023, outlines the design and testing of a lipid nanoparticle (LNP)-encapsulated mRNA vaccine targeting outer surface protein A (OspA) of B. burgdorferi.
OspA is a promising vaccine target because of its critical role in the bacterium’s lifecycle. The research team sought to overcome previous challenges associated with Lyme disease vaccination by leveraging the mRNA-LNP platform, which has proven highly effective in combating viral pathogens like SARS-CoV-2. This approach offers advantages in scalability, rapid production, and the ability to induce potent immune responses.
Research Method
The researchers selected OspA as the focal antigen due to its conservation across diverse B. burgdorferi strains and its essential role in anchoring the bacterium to the tick midgut. Targeting OspA ensures the vaccine can act early in the transmission process, potentially blocking infection before the bacterium is transmitted to humans.
The study involved designing an mRNA sequence encoding OspA, which was encapsulated in LNPs to enhance stability and delivery. The immunogenicity and protective efficacy of this vaccine were compared with a more traditional alum-adjuvanted recombinant OspA protein subunit vaccine. Notably, the mRNA-LNP platform was among the first to be applied to a bacterial pathogen, showcasing the versatility of this technology.
To test the vaccine, Balb/c mice were immunized and evaluated for immune responses, including antibody production, T-cell activation, and protection against B. burgdorferi infection. The researchers also conducted a prime-boost regimen to assess the durability of the immune response.
Results
The mRNA-LNP vaccine demonstrated remarkable success in preclinical trials. A single dose elicited robust activation of both CD4+ and CD8+ T cells, producing high levels of cytokines associated with immune protection, such as interferon-gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α), and interleukin-2 (IL-2). These responses were significantly stronger compared to the alum-adjuvanted protein subunit vaccine.
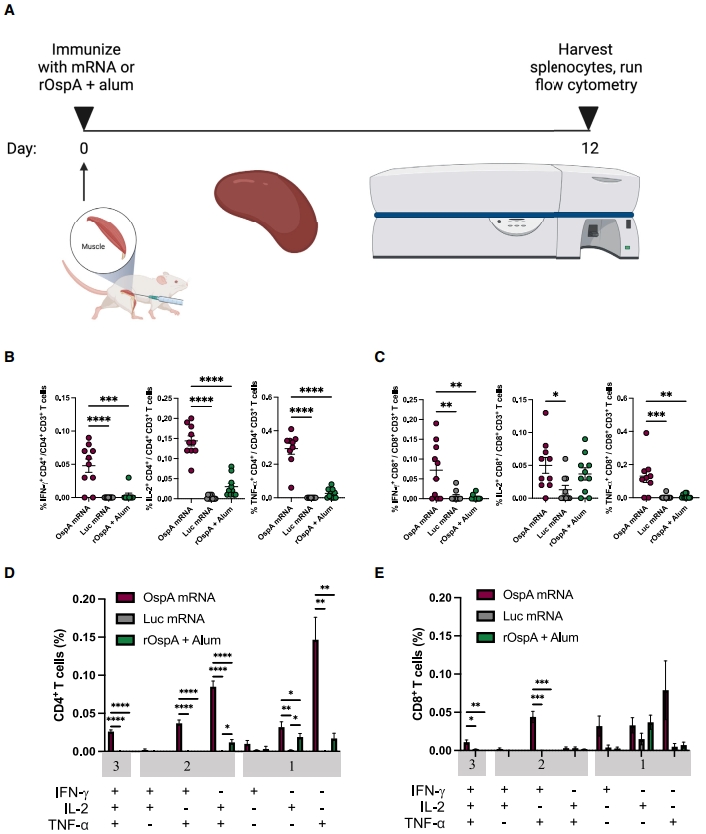
Additionally, the vaccine induced robust germinal center B-cell responses and T follicular helper (Tfh) cell activity in draining lymph nodes. These are critical for generating long-lasting, high-affinity antibodies. The OspA mRNA-LNP vaccine produced significantly higher titers of OspA-specific antibodies compared to the protein vaccine, and these antibodies persisted for at least 24 weeks after vaccination.
When tested in a mouse model of Lyme disease, 80% of animals immunized with the mRNA-LNP vaccine were protected from infection with B. burgdorferi, as evidenced by low bacterial burdens in critical tissues such as the heart, bladder, and skin. In comparison, only 50% of mice vaccinated with the protein subunit vaccine achieved similar protection. This highlights the superior efficacy of the mRNA-LNP approach.
Conclusion
This research represents a significant advancement in Lyme disease prevention. The mRNA-LNP vaccine targeting OspA not only provides strong immune responses but also demonstrates high efficacy and durability in preventing B. burgdorferi infection. The findings emphasize the versatility of the mRNA-LNP platform, which has primarily been applied to viral targets, and its potential to address unmet needs in bacterial vaccine development.
If translated into successful human clinical trials, this vaccine could become the first FDA-approved Lyme disease vaccine in over two decades. Beyond Lyme disease, this study underscores the potential of mRNA-based vaccines to combat other bacterial infections, opening a new frontier in infectious disease prevention.
Reference:
Pine, M., Arora, G., Hart, T.M., Bettini, E., Gaudette, B.T., Muramatsu, H., Tombácz, I., Kambayashi, T., Tam, Y.K., Brisson, D. and Allman, D., 2023. Development of an mRNA-lipid nanoparticle vaccine against Lyme disease. Molecular Therapy, 31(9), pp.2702-2714
