Introduction: Navigating the Challenges of Inflammatory Bowel Disease
Inflammatory bowel diseases (IBDs), including ulcerative colitis (UC) and Crohn’s disease (CD), present persistent challenges due to their chronic nature and significant impact on patients’ quality of life. Despite advancements, current therapies—such as anti-inflammatory drugs and immunosuppressants—often fail to provide long-term relief or address the multifaceted pathology of IBD. Emerging microbiota-based therapies like probiotics and fecal transplants have shown promise but face limitations, including poor clinical outcomes and safety concerns regarding live bacterial strains.
In response to these challenges, a groundbreaking innovation, probiotic-inspired nanomedicine (SeM@EM), has emerged. This hybrid nanotechnology integrates a bioactive diselenide-bridged mesoporous silica core (SeM) with a probiotic-derived Escherichia coli Nissle 1917 (EcN) membrane coating. The result is a safe, multifunctional therapy targeting IBD’s root causes by regulating redox balance, modulating immune responses, and reconfiguring the gut microbiome. This novel approach leverages both biomimetic and nanotechnology advances to address the complex needs of IBD patients.
Research Objective: Advancing Holistic IBD Treatment

The research team behind this breakthrough consists of a collaboration among leading scientists from institutions like the National Center for Nanoscience and Technology, Chinese Academy of Sciences, South China University of Technology, and Columbia University. Their findings, published in Advanced Materials in December 2022, showcase the efficacy of SeM@EM in preclinical models of colitis.
This study underscores the potential of SeM@EM as a comprehensive treatment for IBD, targeting multiple pathological factors such as oxidative stress, immune dysregulation, and microbial imbalances. By combining nanotechnology and biomimicry, the team aims to establish a new paradigm for gastrointestinal disease therapy.
Experimental Process: Building and Validating a Probiotic-Inspired Nanomedicine
The SeM@EM nanomedicine was engineered using a two-component approach: a diselenide-bridged mesoporous silica nanoparticle (SeM) core for antioxidative activity and a probiotic-derived Escherichia coli Nissle 1917 (EcN) membrane coating to enable immune modulation and adhesion to intestinal tissues. The membrane coating was achieved through an ultrasonic assembly process, ensuring stability and functionality in the gastrointestinal environment. Characterization techniques, including transmission electron microscopy and dynamic light scattering, confirmed the core-shell structure and robust performance of SeM@EM in varying pH conditions.
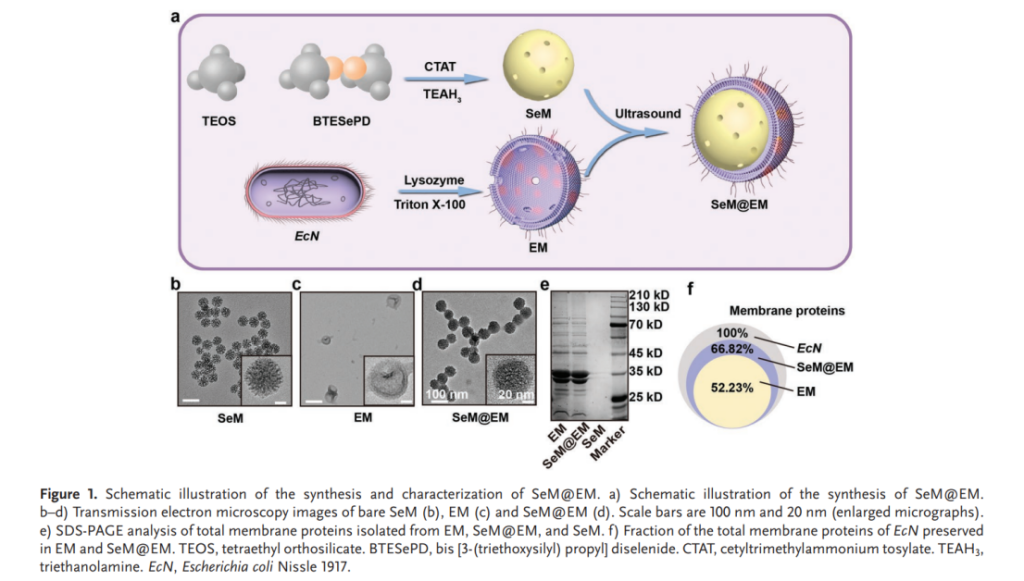
Key findings from preclinical trials in a murine model of DSS-induced colitis demonstrated SeM@EM’s exceptional therapeutic efficacy:
- Redox Balance Restoration: SeM@EM significantly reduced oxidative stress markers, such as reactive oxygen species (ROS) and malondialdehyde (MDA), while enhancing antioxidant enzyme superoxide dismutase (SOD) activity.
- Immune Modulation: The nanomedicine shifted immune responses towards an anti-inflammatory profile, increasing regulatory T cells (Tregs) and B cells, while decreasing pro-inflammatory cytokines like TNF-α and IL-6.
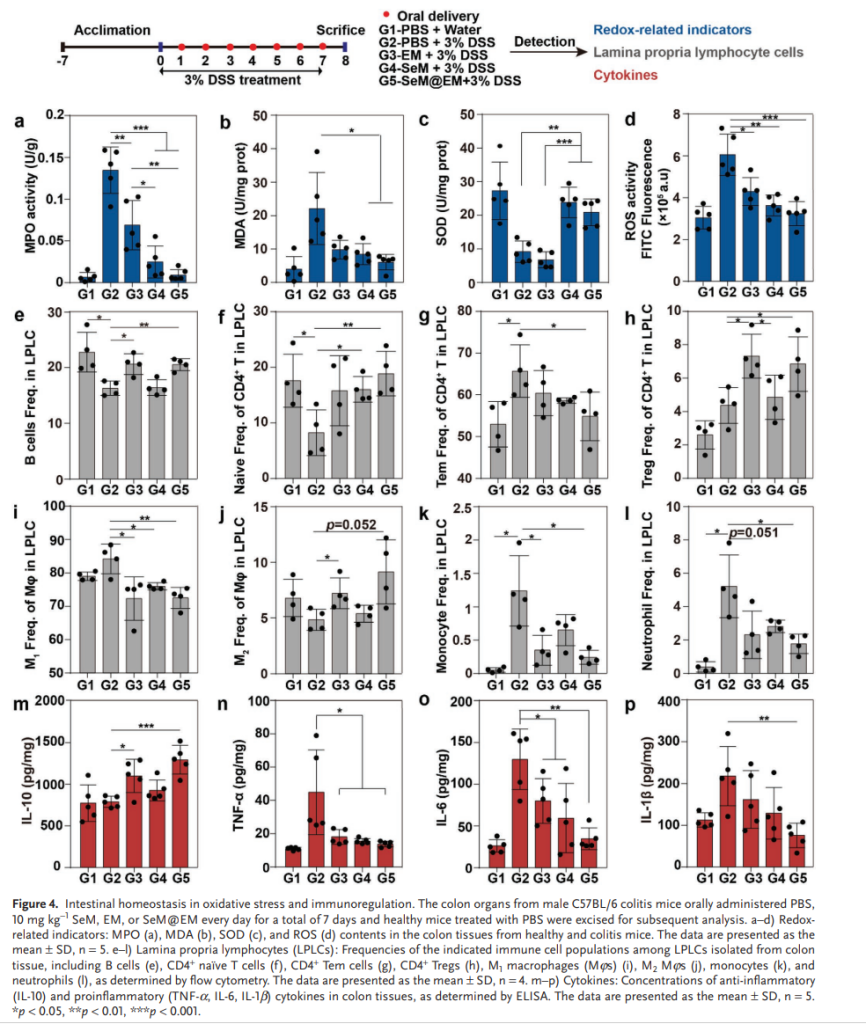
- Gut Microbiome Reconfiguration: SeM@EM restored bacterial diversity and altered the gut microbiome, reducing harmful species like Mucispirillum schaedleri and Bacteroides vulgatus while promoting beneficial short-chain fatty acid (SCFA)-producing bacteria.
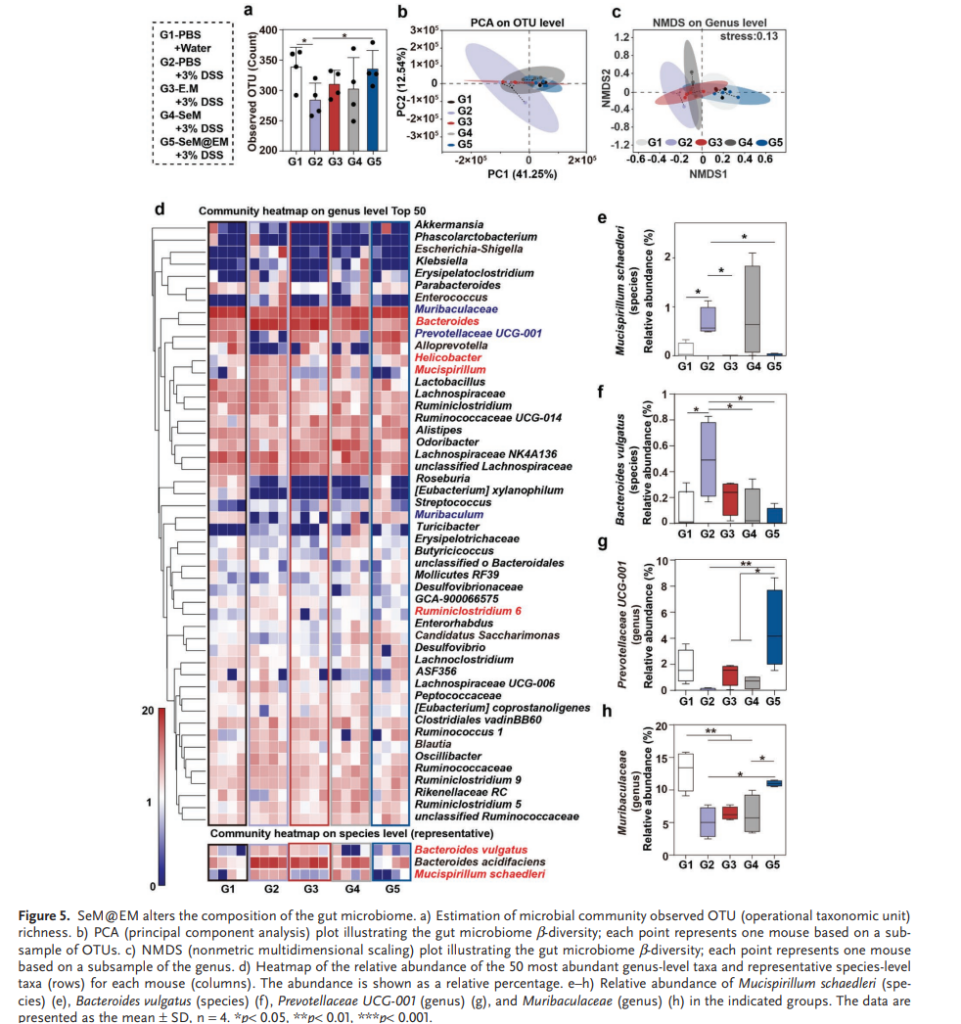
The integration of these functions enabled SeM@EM to outperform traditional therapies in alleviating colitis symptoms. Treated mice exhibited reduced weight loss, lower disease activity indices, and improved colon tissue health. Notably, SeM@EM’s adhesion to the mucus layer prolonged its retention in the colon, enhancing its therapeutic effects.
This multifunctional approach demonstrates SeM@EM’s capacity to address the complex pathology of IBD by simultaneously targeting oxidative stress, immune dysregulation, and microbial imbalance.
Conclusion: A New Horizon in Gastrointestinal Health
The study demonstrated that SeM@EM effectively alleviates colitis symptoms and reestablishes intestinal balance through its multifunctional mechanisms. This innovation not only highlights the therapeutic potential of probiotic-inspired nanomedicines but also underscores the importance of integrating biomimetic designs in addressing complex inflammatory diseases.
While promising, the research acknowledges certain limitations. The study was confined to a murine model of acute colitis, necessitating further validation in other preclinical models and eventual clinical trials. Future investigations could explore SeM@EM as a drug delivery platform or adapt it for other gastrointestinal disorders.
The seamless integration of nanotechnology and microbiota-based therapies in SeM@EM opens new doors for treating IBD and related conditions. With its strong preclinical results and scalable, safe design, SeM@EM may soon become a transformative solution for millions battling chronic inflammatory diseases worldwide.
Reference:
Xu, Jiaqi, et al. “Probiotic‐inspired nanomedicine restores intestinal homeostasis in colitis by regulating redox balance, immune responses, and the Gut Microbiome.” Advanced Materials 35.3 (2023): 2207890.
