
Researchers have developed a novel CSP-LB hydrogel combining MgFe-layered double hydroxide nanosheets with dual growth factors
Clinical Need for Advanced Bone Regeneration Therapies
Bone defects caused by congenital abnormalities, trauma, or pathological conditions significantly impair quality of life. Conventional growth factor-based therapies, such as those using BMP-2, often require high doses to achieve effectiveness, resulting in complications like excessive inflammation, ectopic bone formation, and even increased cancer risk. Current hydrogels, though promising in addressing these challenges, frequently lack adequate mechanical strength and controllable growth factor release capabilities, necessitating further innovation.
Objectives and Collaborative Development of CSP-LB Hydrogel
Research Goal: The study aimed to develop the CSP-LB hydrogel, a thermo-responsive and injectable material that integrates MgFe-layered double hydroxide nanosheets and dual growth factors (BMP-2 and PDGF-BB). Its design allows sequential release of angiogenic and osteogenic factors, addressing both vascularization and bone formation in defect sites.
Contributors and Publication: This research was a collaboration between Peking Union Medical College Hospital and Beijing University of Chemical Technology, published in Advanced Materials (2023).
Detailed Experimental Approach for Hydrogel Development
Fabrication Process
The CSP-LB hydrogel was prepared by incorporating BMP-2-functionalized MgFe-LDH nanosheets into a chitosan-silk fibroin hydrogel matrix loaded with PDGF-BB. Key steps included:
- Synthesis of MgFe-LDH nanosheets: Fabricated via wet-chemical synthesis, yielding nanosheets with uniform 90 nm hexagonal morphology and thicknesses of 3–5 nm.
- BMP-2 Functionalization: BMP-2 was immobilized on MgFe-LDH nanosheets via electrostatic interactions mediated by chondroitin sulfate, achieving over 80% loading efficiency.
- Hydrogel Assembly: The functionalized nanosheets were embedded in the hydrogel matrix, ensuring thermal responsiveness and injectability.
Experimental Methods and Rationale
- Gelation and Mechanical Testing: Gelation time, sol–gel transition temperature, and compression modulus were measured to assess usability and structural stability.
- Biological Evaluation:
In vitro experiments assessed cell migration, angiogenesis, and osteogenesis.
In vivo testing in rabbit skull defect models evaluated bone regeneration efficacy. - Sequential Growth Factor Release: The hydrogel’s capability for a burst release of PDGF-BB and sustained BMP-2 release was quantified using enzyme-linked immunosorbent assay (ELISA).
Scientific Innovations
The sequential release mechanism ensured optimized angiogenesis (via PDGF-BB) followed by osteogenesis (via BMP-2). Additionally, the incorporation of MgFe-LDH nanosheets enhanced mechanical properties while enabling sustained bioactive ion release to support cellular processes.
Key Results Demonstrating Hydrogel Efficacy
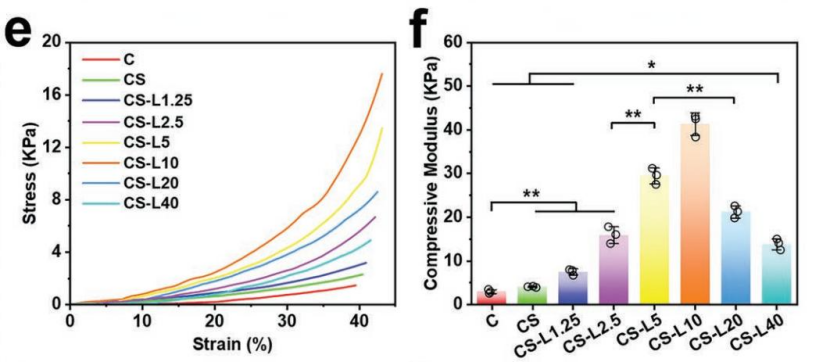
1. Improved Gelation and Mechanical Properties
- Gelation time decreased from 300 to 146 seconds.
- Compression modulus increased 10.2-fold compared to the CS hydrogel.
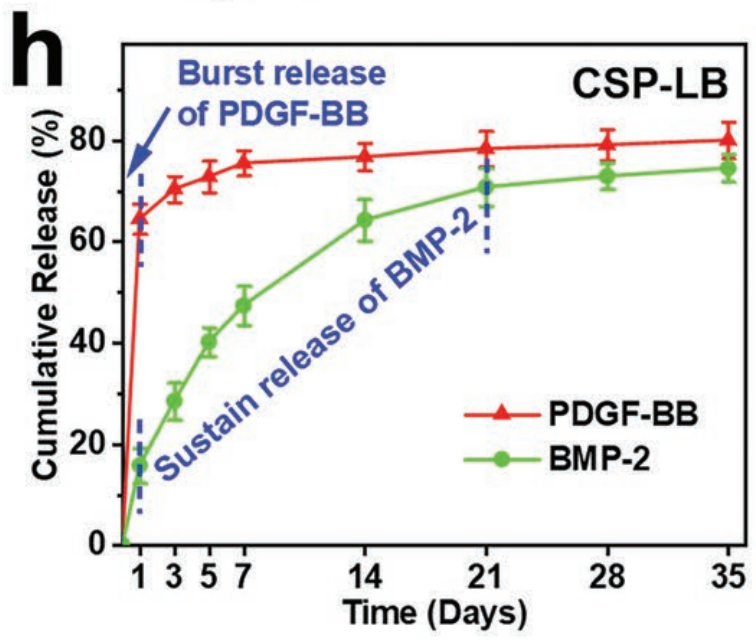
2. Sequential Release Performance
- PDGF-BB exhibited a rapid release with 80.03% released within the first 7 days.
BMP-2 showed sustained release with 74.51% released over 35 days.
3. Enhanced Biological Activity
- Angiogenesis: Migration ratio of endothelial cells increased 10.7-fold. Tube formation showed 2.6-fold increase in junction numbers compared to controls.
- Osteogenesis: Alkaline phosphatase (ALP) activity increased by 4.9-fold, and calcium deposition by 11-fold in CSP-LB compared to CS hydrogel.
4.In Vivo Bone Regeneration
- Rabbit skull defect models revealed a 4.5-fold increase in bone volume and a 3.6-fold increase in mineral density after 8 weeks compared to controls.
Study Significance
This study developed a CSP-LB hydrogel integrating MgFe-LDH nanosheets and dual growth factors, capable of sequential release for bone defect repair. The hydrogel exhibited enhanced gelation and mechanical properties, sustained ion and growth factor release, and significant improvements in angiogenesis and osteogenesis in both in vitro and in vivo settings. These findings highlight its potential for addressing the clinical challenge of effective bone regeneration.
Reference:
Lv, Zehui, et al. “A MgFe‐LDH nanosheet‐incorporated smart thermo‐responsive hydrogel with controllable growth factor releasing capability for bone regeneration.” Advanced materials 35.5 (2023): 2206545.
