A novel in vivo self-assembly strategy has emerged as a game-changing approach to enhancing ferroptosis-based cancer therapy, achieving significant tumor suppression while addressing delivery challenges of current treatments.
Ferroptosis: A New Frontier in Cancer Therapy
Ferroptosis, an iron-dependent, non-apoptotic cell death mechanism, is driven by oxidative stress and the accumulation of lipid peroxides. It has shown tremendous potential in cancer therapy due to its ability to target therapy-resistant tumor cells. At the center of ferroptosis induction lies glutathione peroxidase 4 (GPX4), a key regulator that neutralizes toxic lipid hydroperoxides. Although GPX4 inhibitors are recognized as effective tools for tumor suppression, their clinical application is hampered by poor tumor delivery and nonspecific activation, which reduce therapeutic efficiency and increase side effects. Consequently, innovative drug delivery methods are required to unlock the full potential of ferroptosis-based therapies.
Research Aim & Objectives — A Breakthrough in Tumor-Specific Therapy
The study aimed to overcome the delivery limitations of GPX4 inhibitors and improve their therapeutic efficiency by leveraging a nanotechnology-driven approach. Conducted by a research team led by Da-Yong Hou and colleagues from the National Center for Nanoscience and Technology and Harbin Medical University, the findings were published in Nature Communications (2024). Their solution, a peptide-ferriporphyrin conjugate (Gi-F-CAA), represents a significant step forward in tumor-specific ferroptosis therapy.

Research Method — Harnessing Nanotechnology and Chemistry
The researchers developed Gi-F-CAA, a conjugate designed to self-assemble into nanoparticles in response to the acidic microenvironment of tumors. This conjugate consists of a GPX4 inhibitory peptide linked to a pH-sensitive moiety and ferriporphyrin. The acidic tumor conditions trigger hydrolysis of the pH-sensitive component, leading to self-assembly of the conjugate into nanoparticles. These nanoparticles enhance tumor cell endocytosis, increase binding to GPX4, and induce ferroptosis through the Assembly Enhanced Binding (AEB) effect. This innovative design significantly enhances tumor targeting and reduces off-target effects.
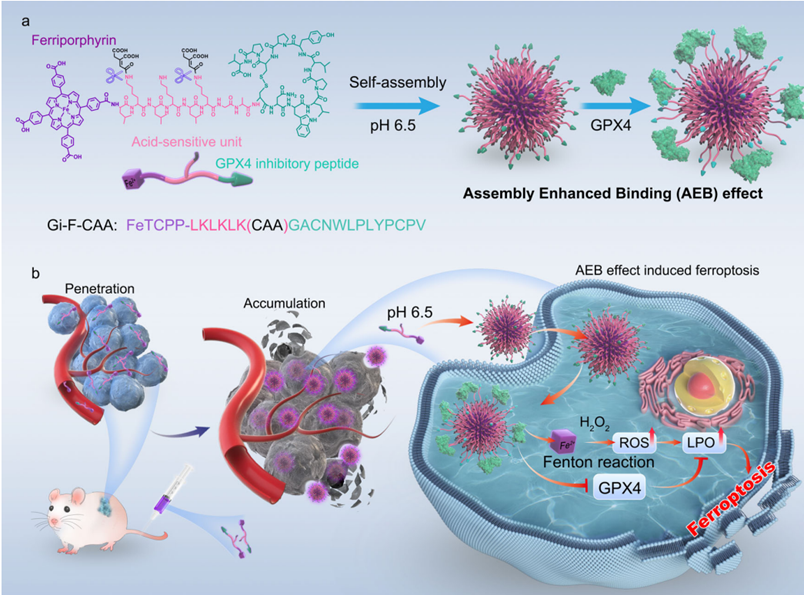
Results — Enhanced Antitumor Activity Through Ferroptosis
The study demonstrated the exceptional potential of Gi-F-CAA in improving ferroptosis-based therapy across multiple tumor models, including bladder cancer, multidrug-resistant breast cancer, and large renal tumors. Key findings include:
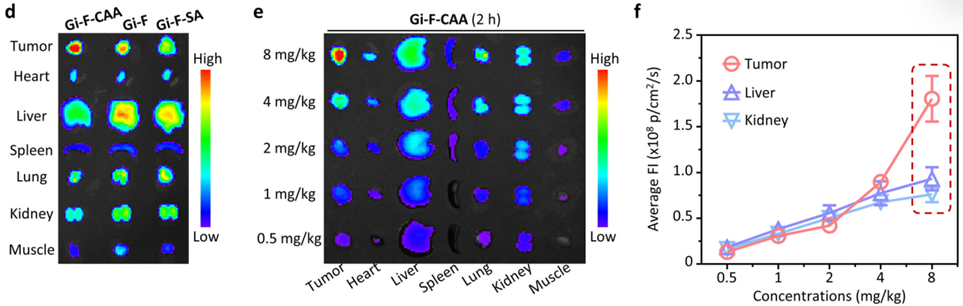
1. Enhanced Tumor Penetration and Accumulation
• Fluorescence imaging results: Gi-F-CAA achieved deep tumor penetration and uniform distribution compared to controls. Quantitatively, fluorescence intensity at the tumor site was ~1.9-fold and ~2.4-fold higher than in the liver and kidneys, respectively, when Gi-F-CAA was administered at a dose of 8 mg/kg.
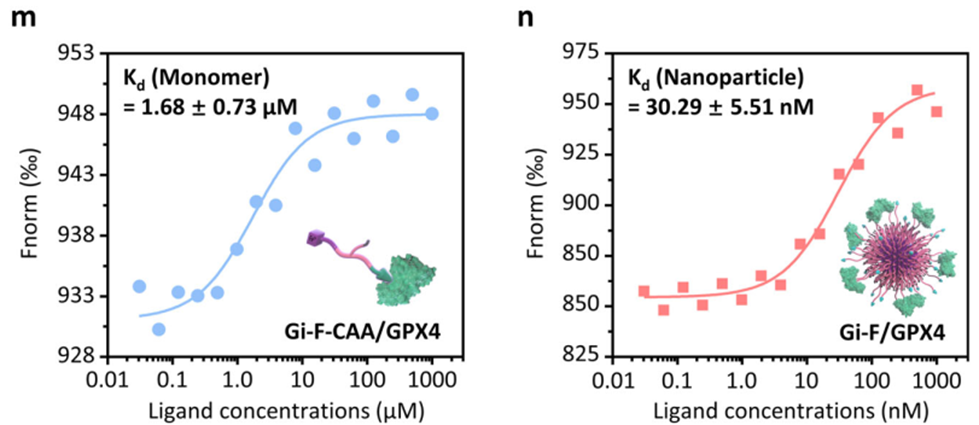
2. Improved GPX4 Inhibition and Oxidative Stress
• Binding Affinity: Gi-F-CAA exhibited a 55-fold higher binding affinity to GPX4 than its single-chain form (Kd: 30.29 nM for nanoparticles vs. 1.68 µM for single chains).
• Biological markers: In treated tumors, GPX4 activity decreased by approximately 2-fold compared to controls, accompanied by a substantial reduction in glutathione (GSH) levels and increased lipid peroxidation (LPO) and reactive oxygen species (ROS) levels.
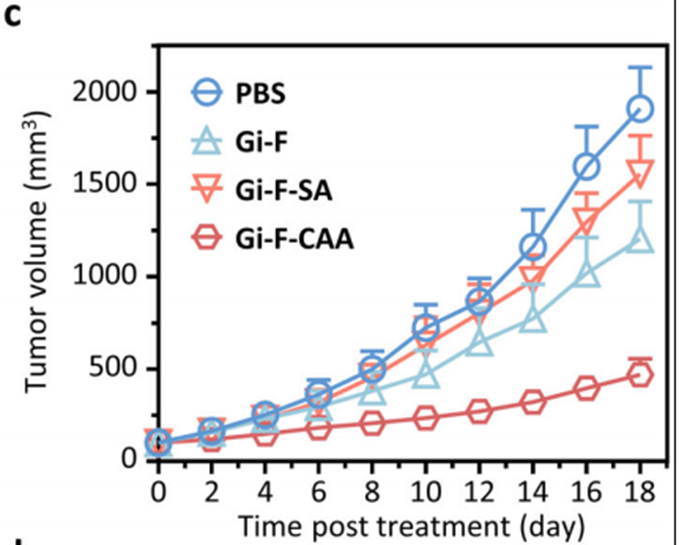
3. Superior Tumor Suppression
• Bladder cancer model: Gi-F-CAA achieved a tumor growth inhibition (TGI) rate of 71%, significantly outperforming controls Gi-F (47%) and Gi-F-SA (38%). The final tumor volume in the Gi-F-CAA group was reduced to 552 mm³, compared to 1,910 mm³ in the PBS-treated control group.
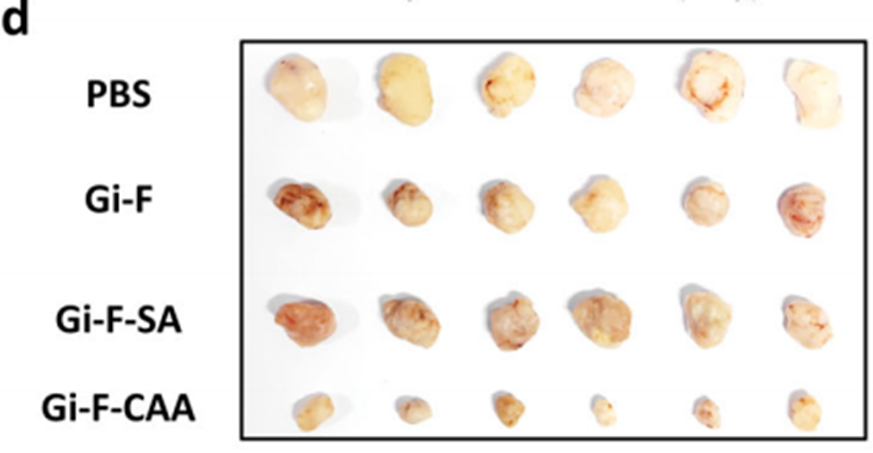
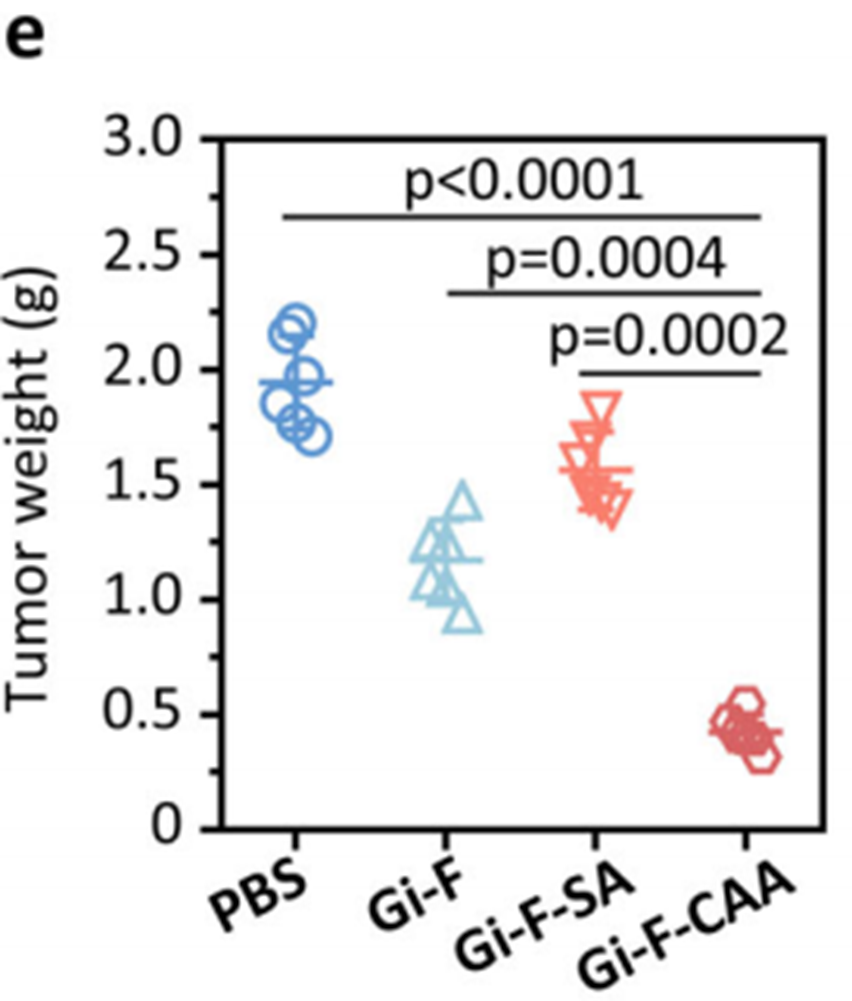
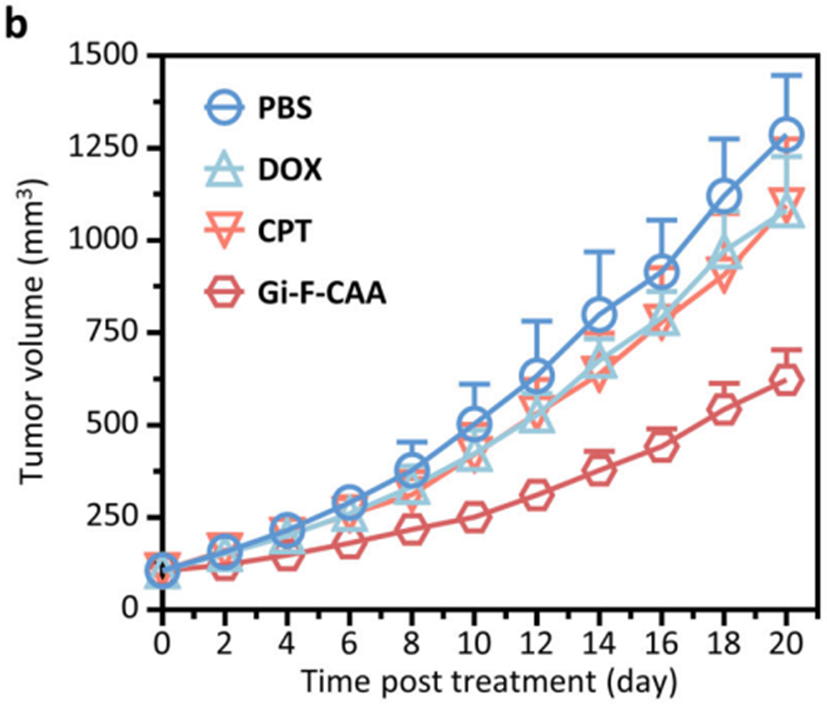
• Multidrug-resistant breast cancer model: Gi-F-CAA-treated tumors exhibited a mean volume of 623 mm³ (TGI rate: 66%), compared to 1,287 mm³ in the PBS group.
• Large renal tumor model: Gi-F-CAA demonstrated sustained tumor growth inhibition, maintaining reduced tumor volumes over time. In contrast, standard antibody-based therapy (Bevacizumab) showed only temporary effects, with tumor growth eventually escaping control.
The evidence collectively highlights Gi-F-CAA’s capacity to overcome critical clinical challenges such as multidrug resistance, poor tumor delivery, and insufficient therapeutic efficacy in large tumors.
Conclusion — Towards New Horizons in Cancer Treatment
The self-assembling Gi-F-CAA conjugate demonstrates substantial potential in ferroptosis-based cancer therapy. By leveraging in vivo self-assembly and the Assembly Enhanced Binding (AEB) effect, this innovative approach enhances tumor penetration, GPX4 inhibition, and oxidative stress, leading to significant tumor suppression in multiple models. These findings provide an alternative strategy for overcoming challenges in malignant tumor therapy, particularly in addressing chemoresistance and improving treatment outcomes. While current research has achieved notable progress, there are still no clinically approved drugs specifically targeting ferroptosis. Despite the limitations, ferroptosis research shows promise for the development of innovative treatments for various diseases. By combination with other therapies, ferroptosis has the potential to improve their efficacy and revolutionize cancer treatment.
Reference:
Hou, Da-Yong, et al. “In vivo assembly enhanced binding effect augments tumor specific ferroptosis therapy.” Nature Communications 15.1 (2024): 454.
