Xi’an, China – A groundbreaking innovation in wound care aims to combat the challenges posed by drug-resistant infections and promote faster healing.
Background
Wound infections caused by drug-resistant bacteria have become a critical healthcare challenge worldwide. These infections not only delay the healing process but also risk severe complications, including chronic inflammation and systemic infection. While antibiotics have traditionally been the mainstay of treatment, their overuse has led to a crisis of antibiotic resistance. Furthermore, most existing wound dressings lack the ability to adapt to the different stages of healing, leaving patients vulnerable to prolonged recovery.
Research Aim & Objectives
To address these issues, researchers have developed a novel self-adaptive wound dressing capable of tailoring its therapeutic actions to different phases of infected wound healing.

A team led by Yutong Yang, Meng Li, Guoying Pan, Jueying Chen, and Baolin Guo from Xi’an Jiaotong University has introduced a new class of wound dressings based on multiple stimuli-responsive cryogels. Published in Advanced Functional Materials in 2023, their research focuses on creating a material that combines advanced antibacterial mechanisms with features that promote healing.
The cryogels are designed to adapt to the wound environment, offering solutions such as antibacterial action during infection, management of biofluids, and support for tissue regeneration in later stages. This development marks a significant step toward overcoming the limitations of conventional therapies.
Research Method
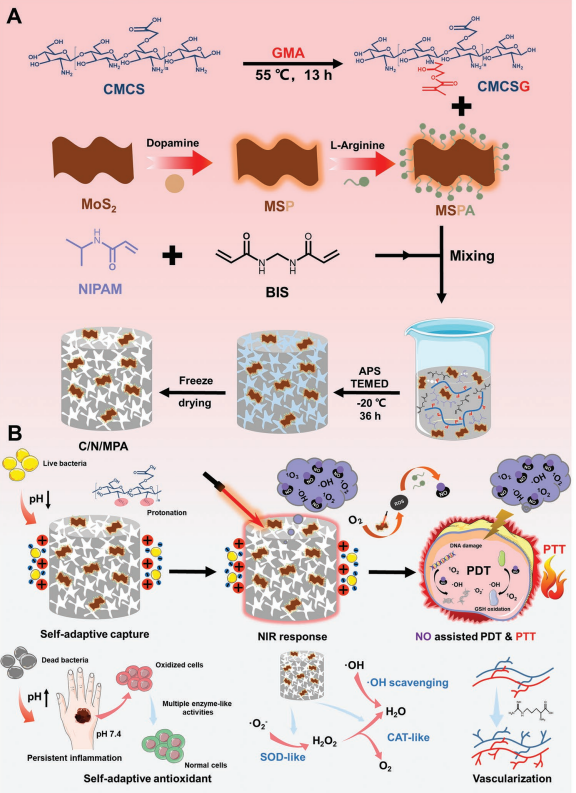
The researchers drew inspiration from previous findings on the therapeutic benefits of nitric oxide (NO) and the enzyme-like activities of MoS₂ nanozymes. Leveraging these properties, they created a multi-functional cryogel using carboxymethyl chitosan (CMCS) and poly(N-isopropylacrylamide) (PNIPAM). These materials were enhanced with MoS₂@polydopamine loaded with L-arginine, forming a system that responds to changes in pH, temperature, and near-infrared (NIR) light.
Key innovations include:
Bacterial capture via pH-responsive protonation: The cryogels actively trap bacteria by changing their surface charge in acidic environments, typical of infected wounds.
NIR-controlled NO release: The cryogels release nitric oxide upon NIR light exposure, boosting their antibacterial efficacy and promoting blood vessel formation.
Enzyme-like activity: The cryogels reduce oxidative stress by mimicking natural enzymes, supporting tissue healing and minimizing inflammation.
Figure 1. Schematic illustration of the preparation and application of multiple stimuli-responsive nanozyme-based cryogel. A) The preparation of CMCSG, MSPA and C/N/MPA; B) Mechanism of multiple stimuli-responsive antibacterial and self-adaptive wound management.
Results
Antibacterial performance: The cryogels eliminated over 98% of MRSA bacterial biofilms within minutes of NIR exposure. This was achieved through a combination of photothermal and photodynamic therapy, enhanced by NO release.
Healing promotion: In animal models, the cryogels accelerated wound closure, with significant improvements in collagen deposition and angiogenesis. Wounds treated with the cryogels healed faster compared to controls, with the NIR-assisted group showing the best outcomes.
Oxidative stress management: The cryogels successfully neutralized harmful reactive oxygen species (ROS), preventing damage to healthy tissue.
This innovative solution not only addresses bacterial infections but also adapts to the evolving needs of the wound during different healing stages, a capability not seen in traditional wound dressings.
Conclusion
The introduction of these self-adaptive cryogels represents a breakthrough in treating infected wounds. By combining multiple functions—antibacterial action, oxidative stress relief, and tissue regeneration—this technology offers a comprehensive approach to wound care.
This innovation demonstrates significant potential for advancing wound management by addressing drug-resistant infections, promoting healing, and reducing inflammation, as evidenced by its successful performance in experimental and preclinical models. For patients suffering from drug-resistant infections, this development brings hope for faster recovery and reduced complications.
Reference:
Yang, Y., Li, M., Pan, G., Chen, J. and Guo, B., 2023. Multiple stimuli‐responsive nanozyme‐based cryogels with controlled NO release as self‐adaptive wound dressing for infected wound healing. Advanced Functional Materials, 33(31), p.2214089.
