Editor: Nina
Scientists investigate a hyaluronic acid-conjugated geraniol nanoconjugate that enhances anti-tumor activity against prostate cancer by promoting mitochondrial dysfunction and apoptosis.
Key Preview
Research Question
This study aims to investigate whether the conjugation of geraniol, a natural compound with anti-cancer properties, to hyaluronic acid (HA) can enhance its efficacy and safety in treating prostate cancer.
Research Design and Strategy
The research employs a systematic approach to evaluate the cytotoxicity of HA-conjugated geraniol (HA-SS-Geraniol) on prostate cancer cells, assess its mechanisms of action, and evaluate its effectiveness in an in vivo xenograft model.
Method
The study utilized various methodologies, including flow cytometry for assessing apoptosis and mitochondrial membrane potential, proteomic analysis for identifying protein expression changes, and in vivo xenograft experiments in nude mice to evaluate anti-tumor efficacy.
Key Results
HA-SS-Geraniol demonstrated significantly increased cytotoxicity against PC-3 prostate cancer cells, evidenced by enhanced apoptosis and cell cycle arrest. In vivo, HA-SS-Geraniol effectively inhibited tumor growth with a tumor growth inhibitory ratio of 54%.
Significance of the Research
The findings suggest that HA-SS-Geraniol could represent a promising therapeutic agent for prostate cancer, potentially improving treatment outcomes while minimizing side effects associated with conventional chemotherapy.
Introduction and Background
Core Role and Significance
Nanoparticles have emerged as crucial tools in drug delivery systems, particularly in the field of oncology. They enable targeted delivery of therapeutic agents directly to cancer cells, thereby enhancing treatment efficacy and minimizing side effects associated with traditional chemotherapy. Specifically, hyaluronic acid (HA)-based nanoparticles are gaining prominence due to their biocompatibility and ability to selectively bind to CD44 receptors overexpressed in various cancers, including prostate cancer. This targeted approach is vital for developing more efficient cancer therapies that align with the principles of precision medicine.
Key Challenges
Despite the significant advancements in nanoparticle drug delivery, several critical challenges persist in this field. These include the variability in nanoparticle size, shape, and surface properties, which can affect their biodistribution and cellular uptake. Additionally, achieving consistent drug release profiles and stability in physiological conditions remains problematic. The tumor microenvironment’s complexity further complicates targeted delivery, as it can lead to reduced efficacy and unintended systemic exposure. Regulatory considerations for nanoparticle formulations also present hurdles, requiring extensive preclinical and clinical validation to ensure safety and efficacy.
Research Foundation
The foundation of research in nanoparticle drug delivery is robust, encompassing a wide range of studies that investigate various nanoparticle types, including liposomes, polymeric nanoparticles, and conjugated systems like hyaluronic acid and geraniol. Previous studies have demonstrated the potential of these nanoconjugates to enhance drug solubility, stability, and release kinetics. Research has also focused on the mechanisms by which nanoparticles interact with cellular targets, providing insights into optimizing formulations for improved therapeutic outcomes.
Clinical Findings
Recent clinical findings have highlighted the promising applications of nanoparticle-based drug delivery systems in cancer treatment. Several studies have shown that these systems can significantly enhance the therapeutic index of chemotherapeutic agents, leading to better tumor response rates and improved patient outcomes. Notably, the use of HA-conjugated nanoparticles has been associated with increased localization of drugs in tumor tissues and reduced side effects. Ongoing clinical trials continue to explore various nanoparticle formulations, emphasizing their potential to transform cancer therapy by providing safer and more effective treatment options for patients. These findings reinforce the need for continued research in nanoparticle drug delivery, particularly in optimizing formulations for specific cancer types.
Scientific Hypothesis and Research Objectives
Scientific Hypothesis
The conjugation of geraniol, a natural compound with known anti-cancer properties, to hyaluronic acid (HA) via disulfide bonds enhances its therapeutic efficacy against prostate cancer by promoting selective targeting of CD44-overexpressing cancer cells, leading to increased mitochondrial dysfunction and apoptosis while minimizing systemic toxicity.
Research Objectives
- Evaluate Cytotoxicity: To assess the cytotoxic effects of HA-conjugated geraniol (HA-SS-Geraniol) on PC-3 prostate cancer cells compared to free geraniol and hyaluronic acid alone, utilizing cell viability assays.
- Investigate Mechanisms of Action: To identify the mechanisms by which HA-SS-Geraniol induces cell death, specifically focusing on mitochondrial membrane potential loss, apoptosis induction, and cell cycle arrest through flow cytometry and proteomic analyses.
- Assess In Vivo Efficacy: To evaluate the anti-tumor efficacy of HA-SS-Geraniol in vivo using subcutaneous xenograft tumor models in nude mice, measuring tumor growth inhibition and safety profiles.
- Characterize Proteomic Changes: To conduct a comprehensive proteomic analysis to identify differentially expressed proteins and biological pathways affected by HA-SS-Geraniol treatment, providing insights into the molecular mechanisms underlying its anti-cancer effects.
- Optimize Formulation for Clinical Application: To explore the formulation and delivery parameters of HA-SS-Geraniol, aiming to enhance its therapeutic potential and establish a foundation for future clinical applications in targeted cancer therapy.
Through these objectives, the study aims to establish HA-SS-Geraniol as a promising candidate for targeted prostate cancer therapy, paving the way for further research and potential clinical translation.
Experimental Basis and Core Findings
Multiple Experimental Foundations
- Cytotoxicity Assessment:
The cytotoxic effects of HA-SS-Geraniol were evaluated using the Cell Counting Kit-8 (CCK-8) method. This assay measures the metabolic activity of living cells, allowing for the quantification of cell viability following treatment with varying concentrations of HA-SS-Geraniol.HA-SS-Geraniol exhibited significantly increased cytotoxicity against PC-3 prostate cancer cells, with an IC50 value of 425.3 μM, compared to free geraniol (IC50 of 13.95 mM). This finding validates the hypothesis that conjugation enhances the anti-cancer effects.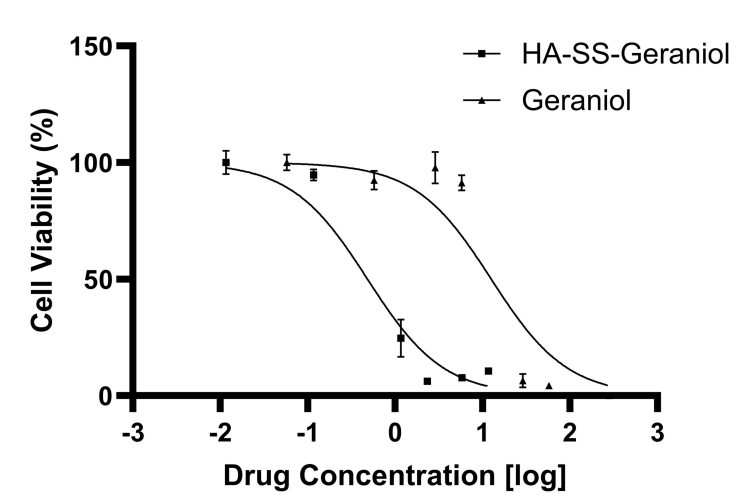
Figure 1. Cell viability analysis of PC-3 cells after treatment with geraniol or HA-SS-geraniol for 48 hours. (Mean ± SD; n = 3; * p < 0.05).
- Mitochondrial Membrane Potential Loss:
Flow cytometry was employed to assess the loss of mitochondrial membrane potential using the Rhodamine 123 (Rh-123) staining method. This technique allows for the visualization of changes in mitochondrial function, which is indicative of early apoptotic events.Treatment with HA-SS-Geraniol resulted in a marked increase in mitochondrial membrane potential loss, with rates rising to 21.90% in the HA-SS-Geraniol group. This supports the hypothesis that HA-SS-Geraniol induces mitochondrial dysfunction.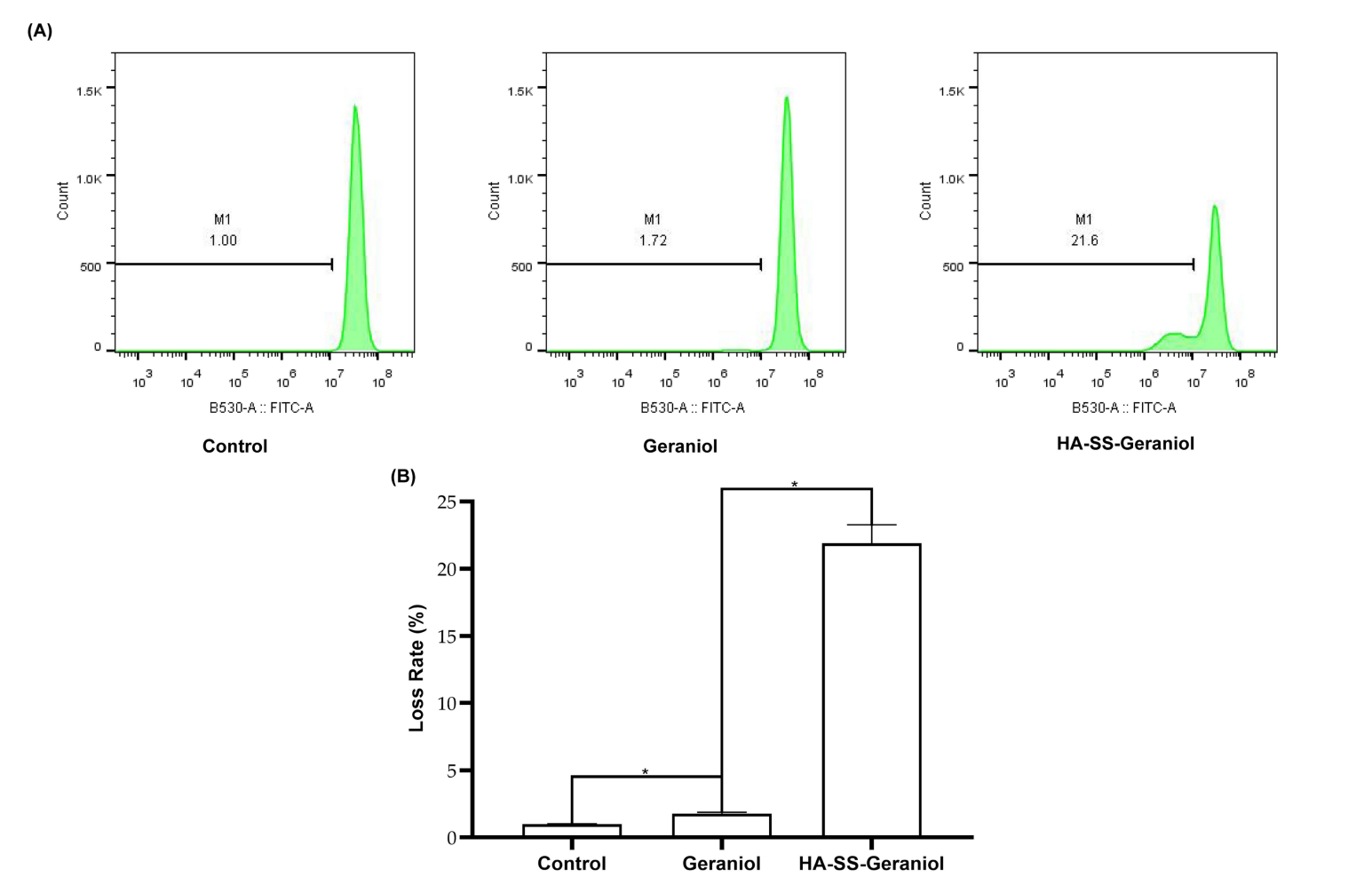
Figure 2. Representative results of mitochondrial membrane potential loss in PC-3 cells after 2 mM geraniol and HA-SS-geraniol treatments by ffow cytometry (A) and statistical analysis of membrane potential loss (Mean ± SD; n = 3; * p < 0.05) (B).
- Apoptosis Induction:
The induction of apoptosis was evaluated using the Annexin V-FITC Apoptosis Detection Kit, which differentiates between early and late apoptotic cells. This method utilizes flow cytometry to quantify the percentage of apoptotic cells in treated populations.Flow cytometry results revealed an apoptotic rate of 48.50% following HA-SS-Geraniol treatment, significantly higher than the control (5.54%) and geraniol (5.13%) groups. This finding confirms the effective induction of apoptosis by HA-SS-Geraniol.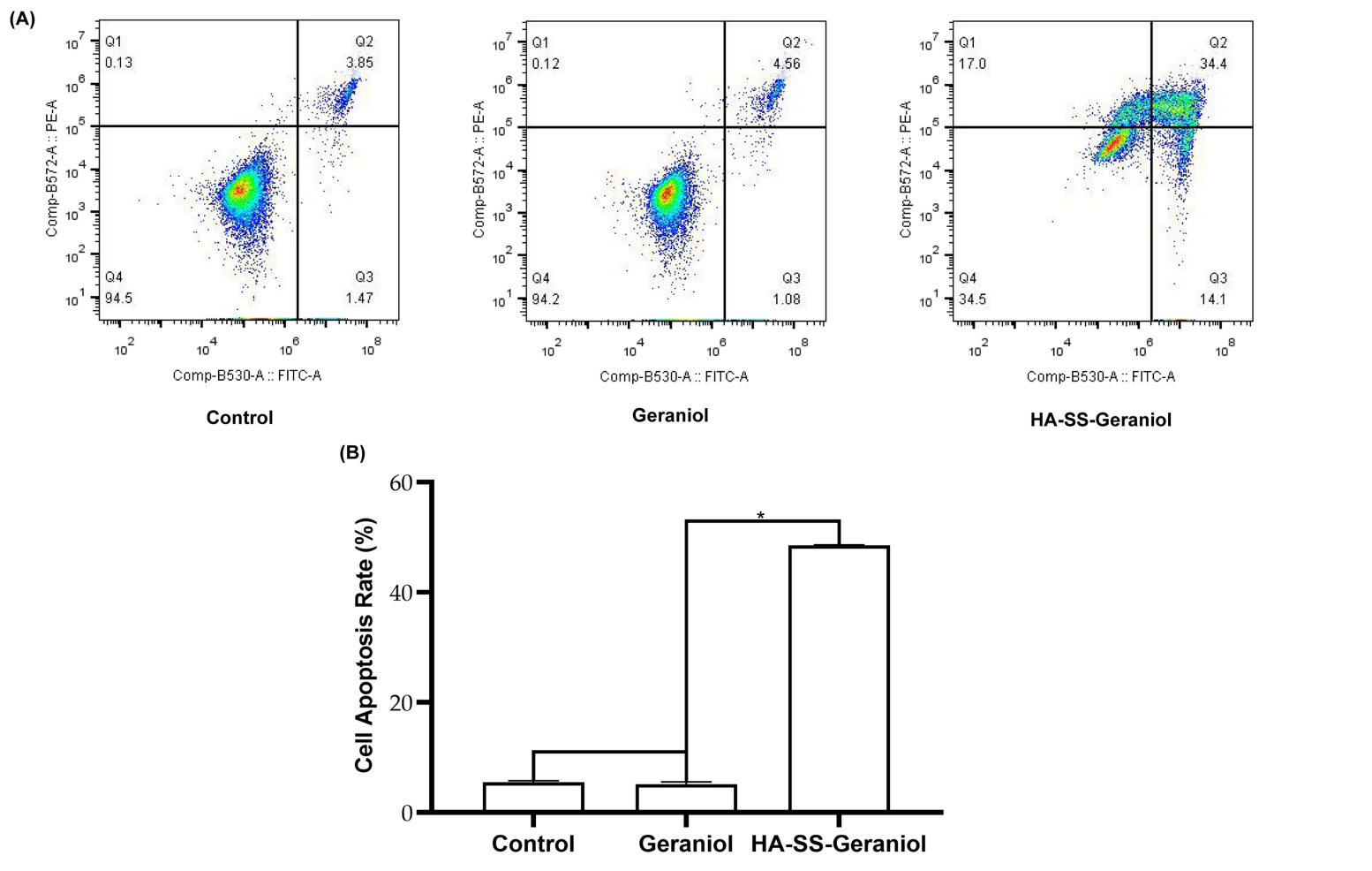
Figure 3. Representative results of apoptotic induction in PC-3 cells after 2 mM geraniol and HA-SS-geraniol treatments by ffow cytometry (A) and statistical analysis of apoptosis rate (Mean ± SD; n = 3; * p < 0.05) (B)
- Cell Cycle Analysis:
Flow cytometry was also used to analyze cell cycle distribution in treated cells by staining with propidium iodide (PI). This approach enables the determination of the proportion of cells in different phases of the cell cycle, particularly assessing the G2 phase.Analysis showed that HA-SS-Geraniol treatment increased the proportion of cells in the G2 phase (21.97%) compared to the control (9.55%) and geraniol (9.00%) groups. This validates the objective of demonstrating cell cycle arrest.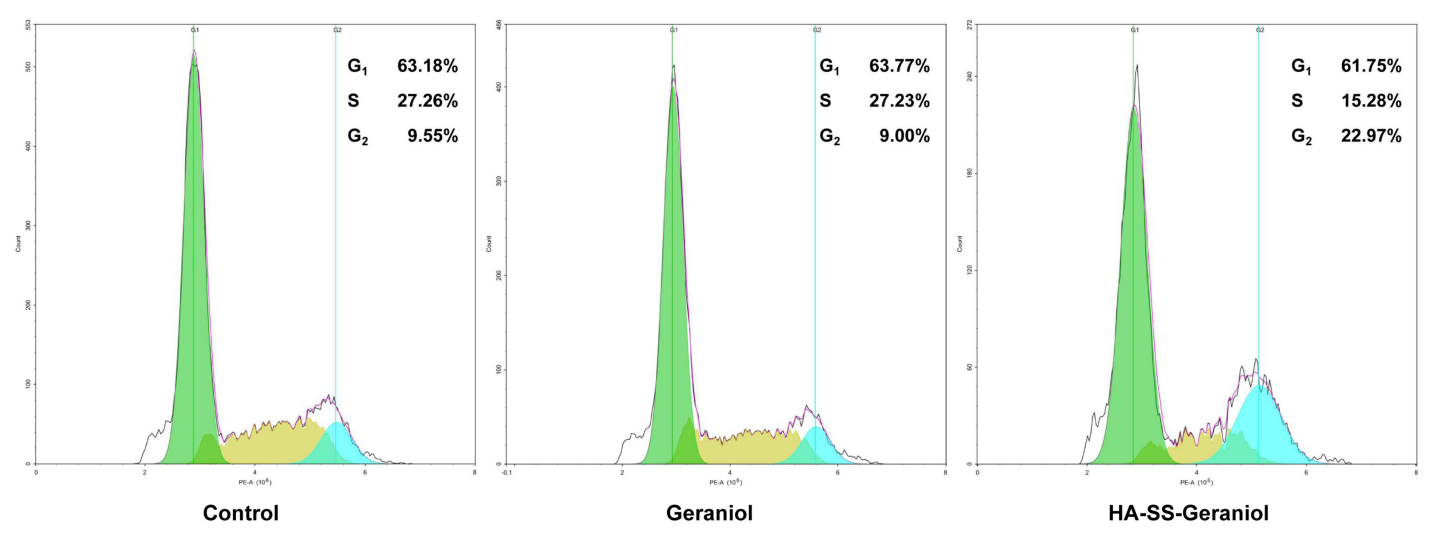
Figure 4. Representative results of cell distribution in PC-3 cells after 2 mM geraniol and HA-SS-geraniol treatments.
- Proteomic Analysis:
A comprehensive proteomic analysis was conducted to identify differentially expressed proteins following treatment with HA-SS-Geraniol. The filter-aided sample preparation (FASP) method was used for protein extraction, followed by LC-MS/MS for protein identification and quantification.Proteomic profiling identified 718 differential proteins, with significant alterations linked to mitochondrial function and apoptosis. This supports the hypothesis regarding the molecular mechanisms of HA-SS-Geraniol’s anti-cancer effects.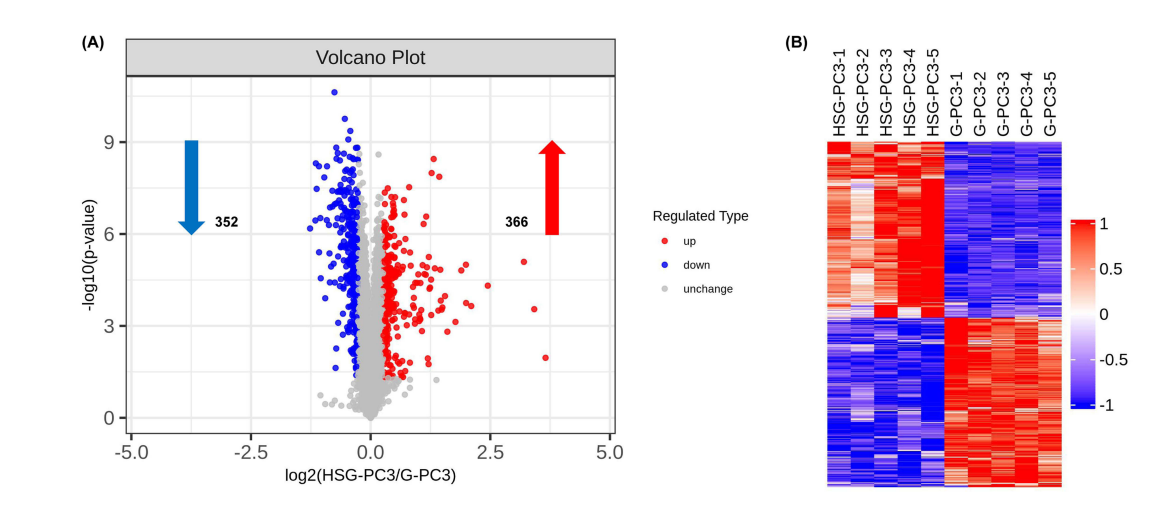
Figure 5. Proteomic response between geraniol and HA-SS-geraniol groups. (A) Volcano plot of differentially expressed proteins, with red and blue dots representing upregulated and down-regulated proteins, respectively. Screening criteria: p value <0.05, Fold change>1.2 or B) Hierarchical clustering of differentially expressed proteins. Red represents an up-regulated protein, blue represents a down-regulated protein, and gray represents no protein quantitative information. Abbreviations: G, Geraniol; HSG, HA-SS-Geraniol.
- In Vivo Efficacy Assessment:
The anti-tumor efficacy of HA-SS-Geraniol was evaluated in a subcutaneous xenograft tumor model using nude mice. Tumor volumes were measured to assess the therapeutic impact of HA-SS-Geraniol in vivo.In vivo studies demonstrated that HA-SS-Geraniol significantly inhibited tumor growth, achieving a tumor growth inhibitory ratio of 54% compared to 9.2% in the geraniol group. This validation reinforces its potential as a targeted cancer therapy.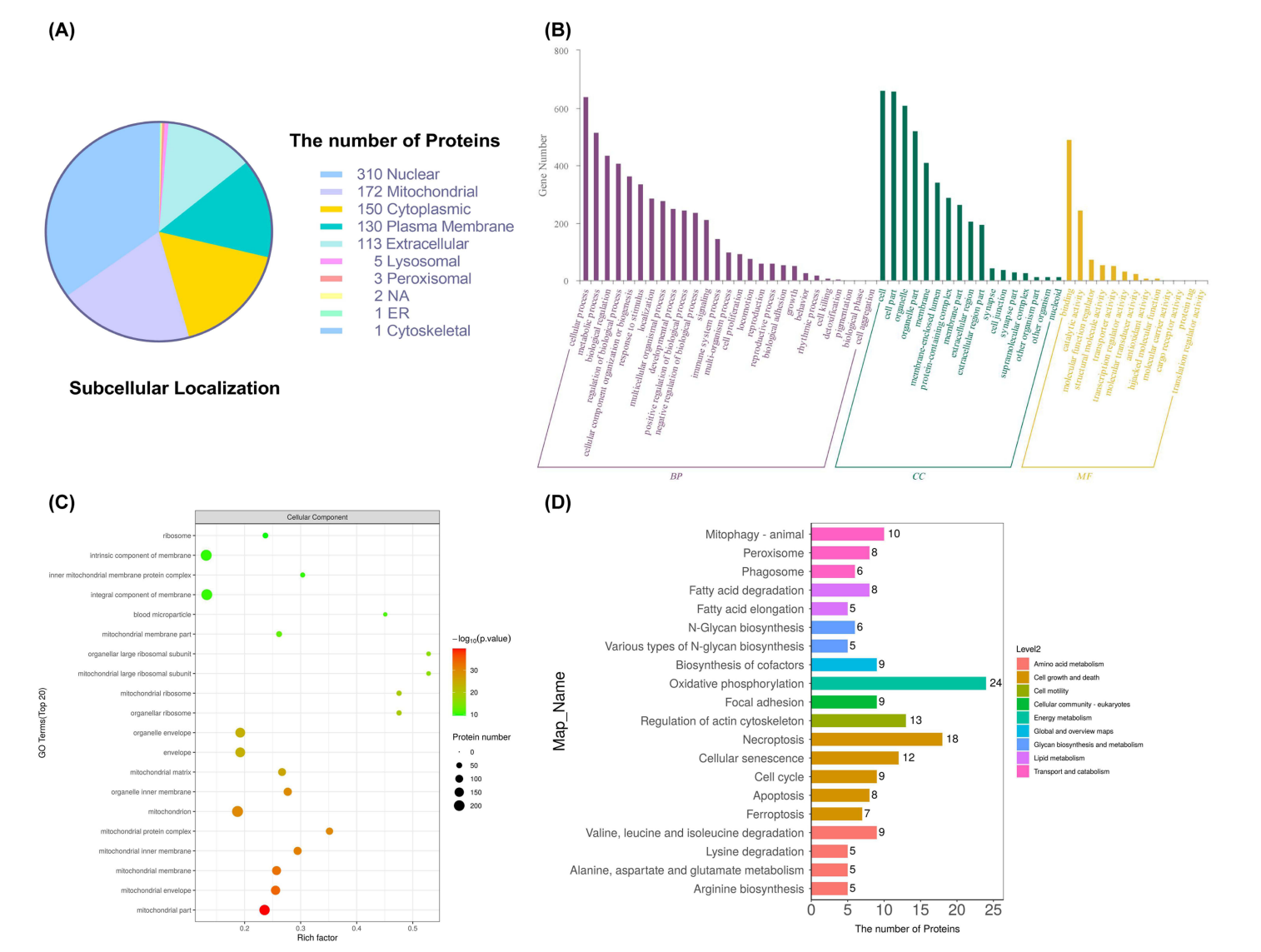
Figure 6. Bioinformatic analysis based on the differentially expressed proteins. (A) Subcellular localization of the differentially expressed proteins. (B) GO annotations of the differentially expressed proteins in biological processes, molecular function, and cellular components. © Enrichment analysis of GO terms in the cellular component. (D) Top 20 annotations of KEGG pathways related to metabolism and cellular processes.
Solution Development: Design and Mechanism of Nanoparticles
Design Concept
The design concept of the nanoparticles revolves around the conjugation of geraniol to hyaluronic acid through disulfide bonds. This structure aims to enhance selective targeting of CD44-overexpressing prostate cancer cells while facilitating controlled drug release in the tumor microenvironment.
Construction Methods
HA-SS-Geraniol nanoparticles were synthesized through a two-step process involving the formation of disulfide bonds between geraniol and hyaluronic acid. The synthesis included the activation of carboxylic acid groups in HA, followed by the coupling of geraniol using a suitable crosslinking agent. This method ensures that the resulting nanoparticles maintain stability while allowing for targeted release.
Mechanism Validation
Mechanism validation for HA-SS-Geraniol involved demonstrating dual targeting through induced proximity and degradation effects. The disulfide bonds were shown to be cleaved in the presence of glutathione, a characteristic of the tumor microenvironment, leading to localized drug release. Additionally, studies indicated that the induced apoptosis was dependent on autophagy pathways, further validating the mechanism of action.
In Vitro Studies
Efficacy Validation
Efficacy validation in vitro involved treating PC-3 prostate cancer cells with HA-SS-Geraniol and evaluating cell viability, apoptosis, and cell cycle distribution. The significant increase in apoptosis and cytotoxicity supports the efficacy of the nanoparticles in targeting cancer cells.
Safety Assessment
In vitro safety assessments were conducted using normal human cell lines to evaluate any cytotoxic effects of HA-SS-Geraniol compared to control treatments. The results indicated a favorable safety profile, with minimal toxicity observed in non-cancerous cells.
Synergistic Effects and Results
The study investigated the synergistic effects of HA-SS-Geraniol in combination with conventional chemotherapeutics. Results demonstrated enhanced anti-cancer effects when HA-SS-Geraniol was used alongside these agents, indicating potential for combination therapy approaches.
In Vivo Efficacy Validation
The efficacy of HA-SS-Geraniol was validated in vivo using different treatment regimens, including:
- Therapy 1: Control group receiving no treatment.
- Therapy 2: Mice treated with geraniol alone.
- Therapy 3: Mice treated with HA-SS-Geraniol.
Results showed that the HA-SS-Geraniol group exhibited a significant reduction in tumor volume compared to both the control and geraniol groups, reinforcing its therapeutic potential.
Mechanistic Exploration
In-Depth Mechanistic Exploration
In-depth exploration of the mechanisms revealed that HA-SS-Geraniol induces apoptosis primarily through mitochondrial dysfunction, leading to the release of cytochrome c and activation of caspases. These findings were supported by proteomic analyses indicating changes in protein expression linked to apoptosis pathways.
Other Potential Mechanisms
Other potential mechanisms include modulation of cellular signaling pathways involved in cell growth and survival, as well as the induction of oxidative stress that may contribute to tumor cell death. The proteomic data indicated alterations in proteins associated with oxidative phosphorylation and energy metabolism.
Summary
The mechanistic research underscores the multifaceted effects of HA-SS-Geraniol, highlighting its ability to induce apoptosis through mitochondrial pathways while also affecting cellular metabolism and signaling pathways. These insights are crucial for understanding the therapeutic potential of this nanoconjugate.
Conclusion and Prospects
Summarize Core Work
This research presents the development and characterization of HA-SS-Geraniol nanoparticles, demonstrating their enhanced anti-cancer efficacy against prostate cancer through targeted delivery and apoptosis induction.
Reiterate Key Findings
Key findings include the significant cytotoxicity of HA-SS-Geraniol, its ability to induce mitochondrial dysfunction and apoptosis, and the promising in vivo efficacy observed in xenograft models. The dual targeting mechanism and favorable safety profile further support its potential as a therapeutic agent.
Highlight Mechanisms and Significance
The mechanisms of action, particularly the role of mitochondrial pathways in apoptosis induction, highlight the scientific significance of this research. The application value of HA-SS-Geraniol as a targeted cancer therapy represents a promising advancement in the field of oncology, with potential implications for improving treatment outcomes in prostate cancer patients.
Reference
Yu, Han, et al. “Targeted Delivery of Geraniol via Hyaluronic Acid-Conjugation Enhances Its Anti-Tumor Activity Against Prostate Cancer.” International Journal of Nanomedicine, vol. 19, 2024, pp. 155-169. Dove Medical Press, https://doi.org/10.2147/IJN.S444815.
