Editor: Nina
Scientists develop a microRNA-146a-loaded magnesium silicate nanosphere system to enhance bone regeneration and modulate inflammation in oral-maxillofacial defects.
Key Preview
Research Question
Can microRNA-146a-loaded magnesium silicate nanospheres (MSNs) enhance bone regeneration in an inflammatory environment?
Research Design and Strategy
The study employs a combination of in vitro and in vivo experiments to assess the dual effects of MSN+miR-146a on osteogenic differentiation and macrophage polarization.
Method
Human dental pulp stem cells (hDPSCs) and mouse bone marrow-derived macrophages (BMMs) were utilized to evaluate the effects of the MSN+miR-146a complex on osteogenesis and inflammation. Additionally, a stimulated infected mouse mandibular bone defect model was used for in vivo analysis.
Key Results
The MSN+miR-146a complex promoted osteogenic differentiation in hDPSCs and modulated macrophage polarization, favoring M2-type over M1-type macrophages, leading to enhanced bone regeneration in vivo.
Significance of the Research
This study presents a novel biomaterial approach that addresses the challenge of bone regeneration in inflammatory conditions, potentially offering new avenues for therapeutic strategies in oral-maxillofacial defects.
Introduction
Oral and maxillofacial bone defects can arise from various causes, including trauma, tumor resection, periodontitis, and other inflammatory conditions. These defects pose significant challenges for effective treatment and rehabilitation, as they can lead to functional impairments, aesthetic concerns, and chronic pain. Chronic inflammation, in particular, plays a critical role in hampering the natural healing processes, leading to delayed healing and complications such as nonunion or malunion of fractures. Conditions such as osteoarthritis, diabetes, and rheumatoid arthritis can exacerbate these challenges, making the management of bone regeneration in inflammatory environments a pressing concern in clinical practice.
In traditional treatment strategies, drug delivery is often achieved using systemic administration methods, such as oral or intravenous routes. These conventional methods aim to provide therapeutic agents throughout the body; however, they frequently result in suboptimal drug concentrations at the target site. Additionally, systemic delivery can lead to significant side effects, reduced drug efficacy, and the need for higher doses to achieve the desired therapeutic outcomes, which further complicates patient management.
The challenges faced by current drug delivery approaches include inadequate localization of therapeutic agents, poor bioavailability, and an inability to sustain drug release at the target site. These limitations often result in prolonged healing times, increased risk of complications, and a lack of effective regeneration in complex bone defects. Furthermore, traditional methods do not adequately address the inflammatory microenvironment, which can significantly hinder the regenerative process.
To overcome these challenges, innovative drug delivery strategies are being explored, focusing on localized and targeted delivery systems. One such strategy involves the use of nanotechnology, specifically designed nanoparticles that can encapsulate therapeutic agents and deliver them directly to the site of injury. In this study, magnesium silicate nanospheres (MSNs) are utilized for the loading and controlled release of microRNA-146a (miR-146a), an endogenous small noncoding RNA known for its roles in modulating inflammation and promoting osteogenic differentiation. This approach aims to enhance the therapeutic efficacy of miR-146a while simultaneously addressing the inflammatory conditions that typically impede bone regeneration. By improving the localized delivery of miR-146a, this innovative strategy holds the potential to significantly advance the treatment of oral and maxillofacial bone defects, paving the way for more effective and targeted regenerative therapies.
Research Team and Aim
The research team for this study was led by Jiakang Yang, a prominent investigator in the field of regenerative medicine and tissue engineering. This research was conducted at the Stomatology Hospital, Zhejiang University School of Medicine, during 2023. The team included co-authors Jing Shuai, Lixuen Siow, and several other contributors who provided valuable insights and expertise throughout the study. Their findings were presented in the paper titled “MicroRNA-146a-loaded magnesium silicate nanospheres promote bone regeneration in an inflammatory microenvironment,” which was published in the journal Bone Research.
The primary aim of the research, as articulated by Jiakang Yang, was to fabricate the MSN+miR-146a complex and evaluate its effects on bone regeneration and immune response modulation. This study sought to explore the potential of this innovative biomaterial approach in addressing the challenges of bone regeneration within inflammatory environments, ultimately contributing to more effective therapeutic strategies for oral-maxillofacial defects.
Experimental Process
Experiment 1: Fabrication of MicroRNA-146a-Loaded Magnesium Silicate Nanospheres (MSNs)
Primary Technique
The primary technique used in this study was the synthesis of magnesium silicate nanospheres (MSNs) modified with polyethyleneimine (PEI) for the loading of microRNA-146a (miR-146a). This method underpins the research’s goal of developing a novel nanobiomaterial that effectively delivers miR-146a to enhance bone regeneration in an inflammatory environment.
Key Steps
- Synthesis of MSNs: Monodispersed silica colloidal nanospheres were prepared using the modified Stöber method, resulting in a mean diameter of approximately 200 nm.
- Modification with PEI: MSNs were resuspended in deionized water and mixed with a PEI solution (1 mg/mL) for 3 hours at room temperature to enhance the loading capacity for miR-146a.
- Loading miR-146a: Various weight ratios of MSN to miR-146a (0:1, 25:1, 50:1, 75:1, etc.) were tested to determine the optimal loading capacity, confirmed through gel retardation assays.
Data Collection and Analysis
The study employed scanning electron microscopy (SEM) and transmission electron microscopy (TEM) to analyze the morphology of MSNs. Energy-dispersive spectroscopy (EDS) was conducted to confirm the chemical composition. Zeta potential measurements were utilized to assess surface charge variation corresponding to different loading ratios.
Result
The optimal loading ratio of MSN to miR-146a was determined to be 75:1, which resulted in a positive surface charge conducive for cellular uptake. The successful synthesis of MSNs was confirmed by the retention of miR-146a in gel retardation assays.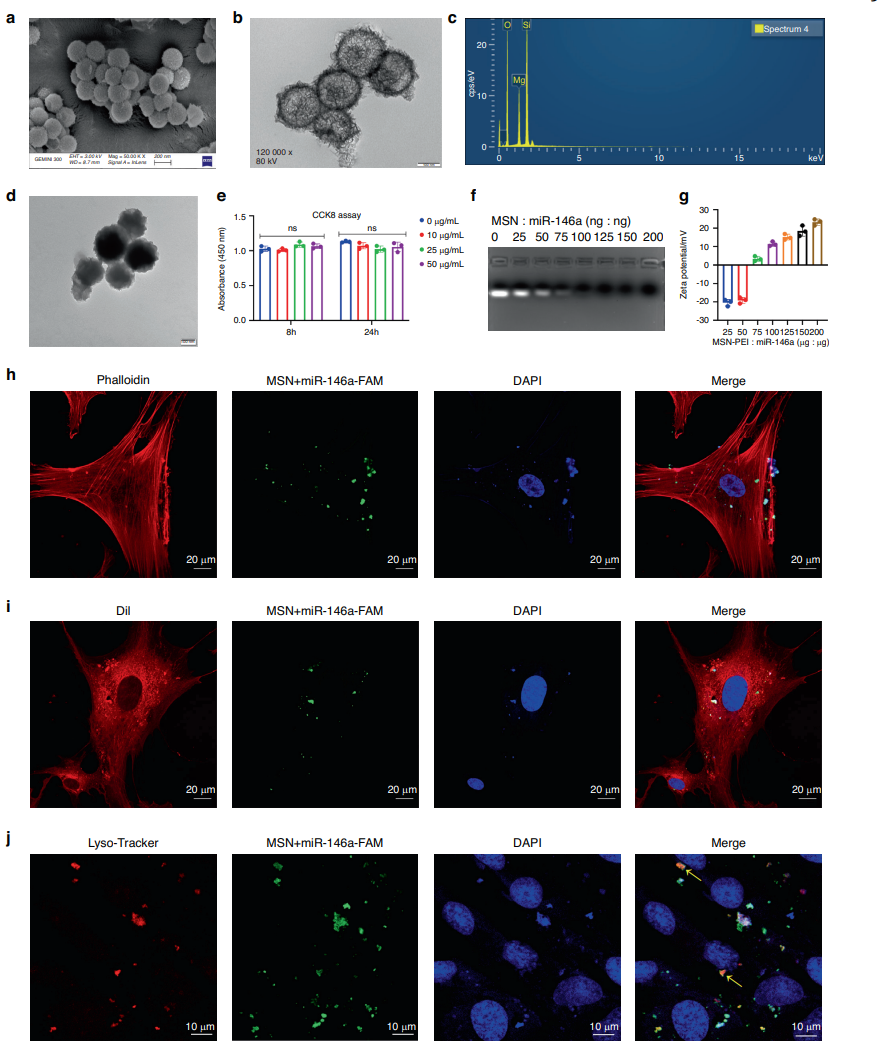
Figure 1. Preparation and characterization of the MSN+miR-146a complex. a–c ECM and TEM images and EDS of MSNs. d TEM images of MSNPEI. e CCK-8 assay of hDPSCs after 8 and 24 h of coculture with PEI-modified MSNs at various concentrations. f, g Gel retardation and zeta potential tests of the MSN+miR-146a complex at different weight ratios. h–j Cellular uptake assay of MSN+miR-146a-FAM in hDPSCs after 24 h of coculture. Cells were stained to label the cytoskeleton, cell membrane or lysosome. Yellow arrows indicate colocalization of lysosomes and MSN+miR-146a-FAM. ns no significance. One-way ANOVA with Dunnett’s multiple comparisons to the blank group was used
Novel Aspect
This study introduces a novel approach to using MSNs as a delivery vehicle for miR-146a, enhancing the loading efficiency and biocompatibility compared to traditional liposomal delivery systems. This modification allows for a more sustained and controlled release of miR-146a, which is crucial for therapeutic applications in bone regeneration.
Experiment 2: In Vitro Evaluation of Osteogenic Differentiation in Human Dental Pulp Stem Cells (hDPSCs)
Primary Technique
The second key experiment involved assessing the osteogenic differentiation of hDPSCs induced by the MSN+miR-146a complex through quantitative assays and staining methods.
Key Steps
- Cell Culture: hDPSCs were isolated and cultured until they reached 80% confluence.
- Transfection: Cells were divided into four groups: miR-146a group, negative control (NC) group, MSN group (loading NC), and MSN+miR-146a group. Each group received the respective treatment for 6 hours.
- Osteogenic Induction: After transfection, cells were cultured in osteogenic medium containing ascorbic acid and β-glycerophosphate for 7 and 14 days, with medium changes every three days.
Data Collection and Analysis
Alkaline phosphatase (ALP) staining and quantitative assays were performed to measure enzyme activity. Mineralization was evaluated using Alizarin red S staining after 14 days. The expression levels of osteogenic markers (COL1A1, RUNX2, and OSX) were quantified using qRT-PCR and Western blotting.
Result
The MSN+miR-146a group exhibited significantly higher ALP activity and enhanced mineralization compared to the other groups, indicating superior osteogenic differentiation. The expression of RUNX2 and OSX was upregulated, corroborating the osteogenic potential of the MSN+miR-146a complex.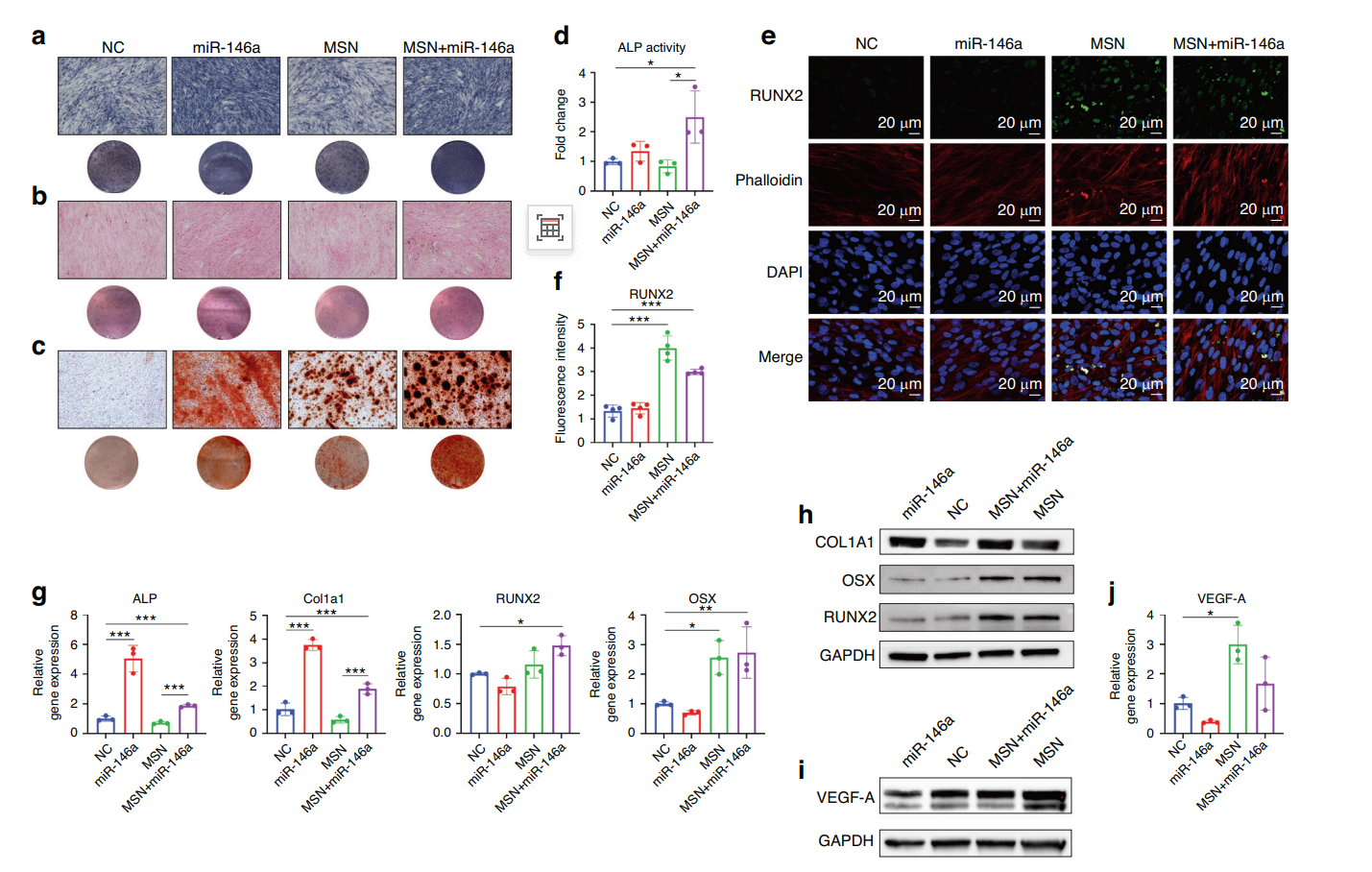
Figure 2. MSNs+miR-146a improved the osteogenic differentiation of hDPSCs. a, b ALP staining and SR staining of hDPSCs after 7 days of osteogenic culture. c ARS staining of hDPSCs after 14 days of osteogenic culture. d Enzyme activity assay of intracellular ALP expression in hDPSCs after 7 days of osteogenic culture. e, f IF images and quantitative analysis (n = 4) of RUNX2 (green) in hDPSCs after 7 days of osteogenic culture. g, h qRT‒PCR (n = 3) and WB results of osteogenic biomarker expression in hDPSCs after 7 days of osteogenic culture. i, j WB and qRT‒PCR (n = 3) results of the expression of VEGF-A in hDPSCs after 24 h of coculture with LPS-stimulated BMM-derived conditioned medium. *P < 0.05, **P < 0.01, ***P < 0.001. One-way ANOVA with Tukey’s multiple comparisons test among all groups was used
Novel Aspect
This experiment demonstrates the synergistic effect of combining MSNs with miR-146a, which effectively enhances the osteogenic differentiation of hDPSCs beyond what is achievable with traditional delivery methods. The sustained release profile of the MSN+miR-146a complex leads to prolonged exposure and activity of miR-146a in promoting osteogenesis.
Experiment 3: In Vitro Polarization of Bone Marrow-Derived Macrophages (BMMs)
Primary Technique
The third experiment focused on the immunomodulatory effects of the MSN+miR-146a complex by evaluating its influence on BMM polarization under inflammatory conditions induced by lipopolysaccharide (LPS).
Key Steps
- Isolation of BMMs: Mouse femur and tibia were used to isolate BMMs, which were cultured in M-CSF for differentiation.
- Transfection: BMMs were transfected with miR-146a or NC using the same methods as hDPSCs.
- LPS Stimulation: After transfection, BMMs were stimulated with LPS (1 μg/mL) for 24 hours to induce pro-inflammatory M1 polarization.
Data Collection and Analysis
Flow cytometry was performed to analyze the surface markers CD40 (M1) and Arg-1/CD163 (M2) to evaluate macrophage polarization. Additionally, qRT-PCR assessed the expression of pro-inflammatory cytokines (IL-1β, IL-6) and anti-inflammatory markers (Arg-1, IL-10).
Result
The results indicated that the MSN+miR-146a complex significantly reduced the proportion of CD40high M1 macrophages and increased Arg-1high M2 macrophages, demonstrating a shift towards an anti-inflammatory phenotype. The expression of IL-1β and IL-6 was significantly downregulated, while Arg-1 levels increased in treated groups.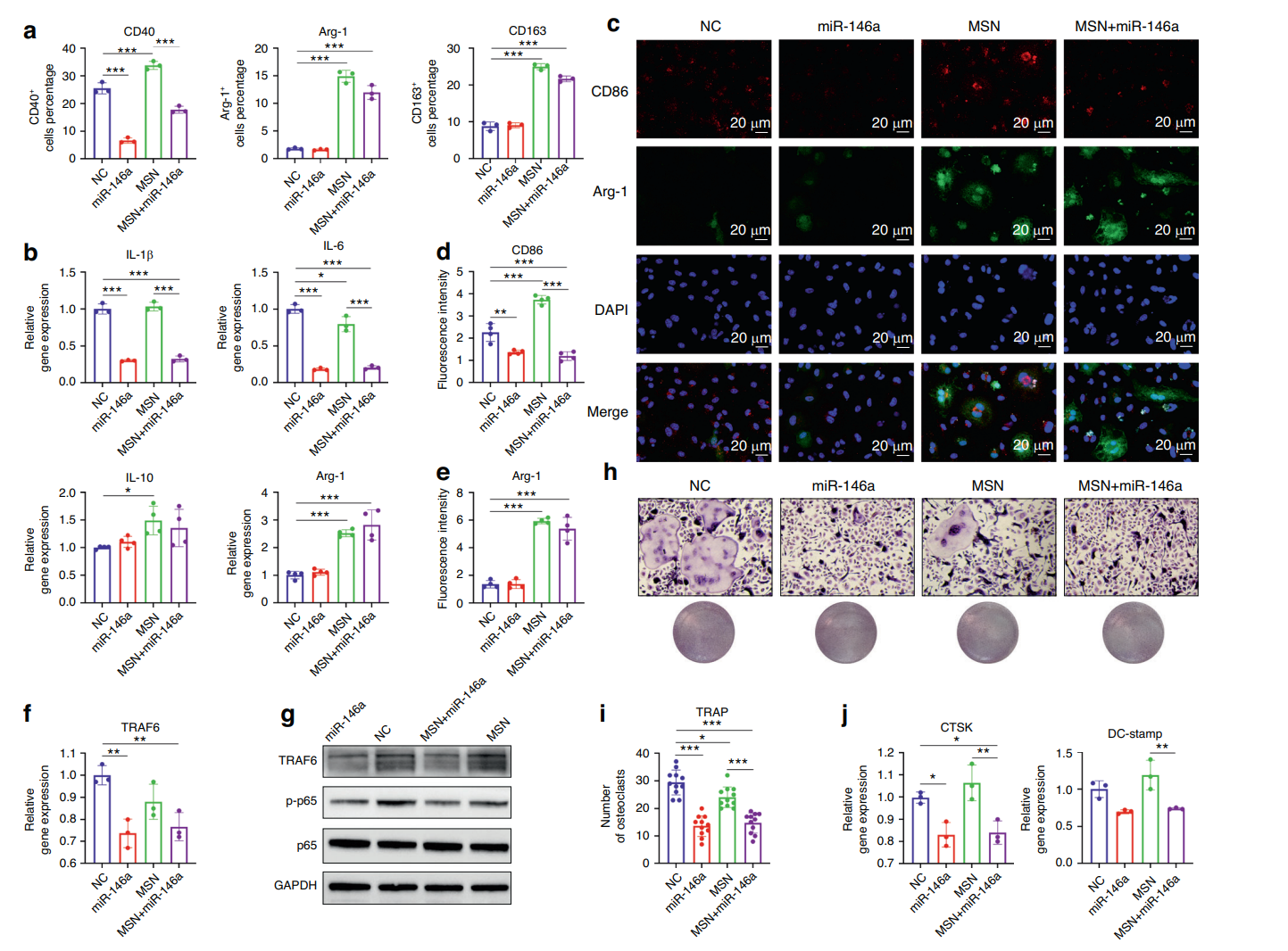
Figure 3. MSNs+miR-146a regulated BMM polarization and osteoclast formation. a Statistical results of flow cytometry of CD40-, Arg-1- or CD163-marked BMMs in the four groups after 24 h of LPS stimulation (n = 3). b The mRNA expression of IL-1β, IL-6, Arg-1 and IL-10 after 24 h of LPS stimulation (n = 3). c–e IF images and quantitative analysis of CD86 (red)- and Arg-1 (green)-marked BMMs after 24 h of LPS stimulation (n = 4). f, g qRT-PCR (n = 3) and WB results of the expression of TRAF6 and phosphorylation level of p65 in BMMs after 24 h of LPS stimulation. h, i TRAP staining and quantitative analysis (n = 11) of osteoclasts in the four groups after 6 days of RANKL induction. j The mRNA expression of CTSK and DC-stamp in osteoclasts after 6 days of RANKL induction (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001. One-way ANOVA with Tukey’s multiple comparisons test among all groups was used
Novel Aspect
This experiment highlights the dual functionality of the MSN+miR-146a complex; not only does it promote osteogenesis, but it also effectively modulates the inflammatory response by skewing macrophage polarization. This presents a significant advancement over traditional delivery systems that typically do not address immune modulation.
Experiment 4: In Vivo Evaluation in Mouse Mandibular Bone Defect Model
Primary Technique
The final experiment involved the in vivo application of the MSN+miR-146a complex in a mouse model of infected mandibular bone defects to assess its effectiveness in promoting bone regeneration.
Key Steps
- Model Creation: A 2 × 1.5 × 1 mm bone defect was created in the mandible of 8-week-old male C57BL/6 mice.
- Biomaterial Application: The MSN+miR-146a complex was delivered via a photocuring hydrogel (GelMA) at the defect site.
- Healing Period: Mice were monitored for 2 and 4 weeks post-surgery, with euthanasia conducted at specified time points.
Data Collection and Analysis
Micro-computed tomography (micro-CT) was used to evaluate bone volume/total volume (BV/TV), bone mineral density (BMD), and trabecular separation (Tb.Sp). Histological analyses and immunofluorescence staining were performed to assess new bone formation and macrophage polarization within the defect area.
Result
At both 2 and 4 weeks, the MSN+miR-146a group showed significantly greater BV/TV and BMD compared to control groups, with histological analysis revealing greater amounts of newly formed bone tissue and reduced osteoclast presence.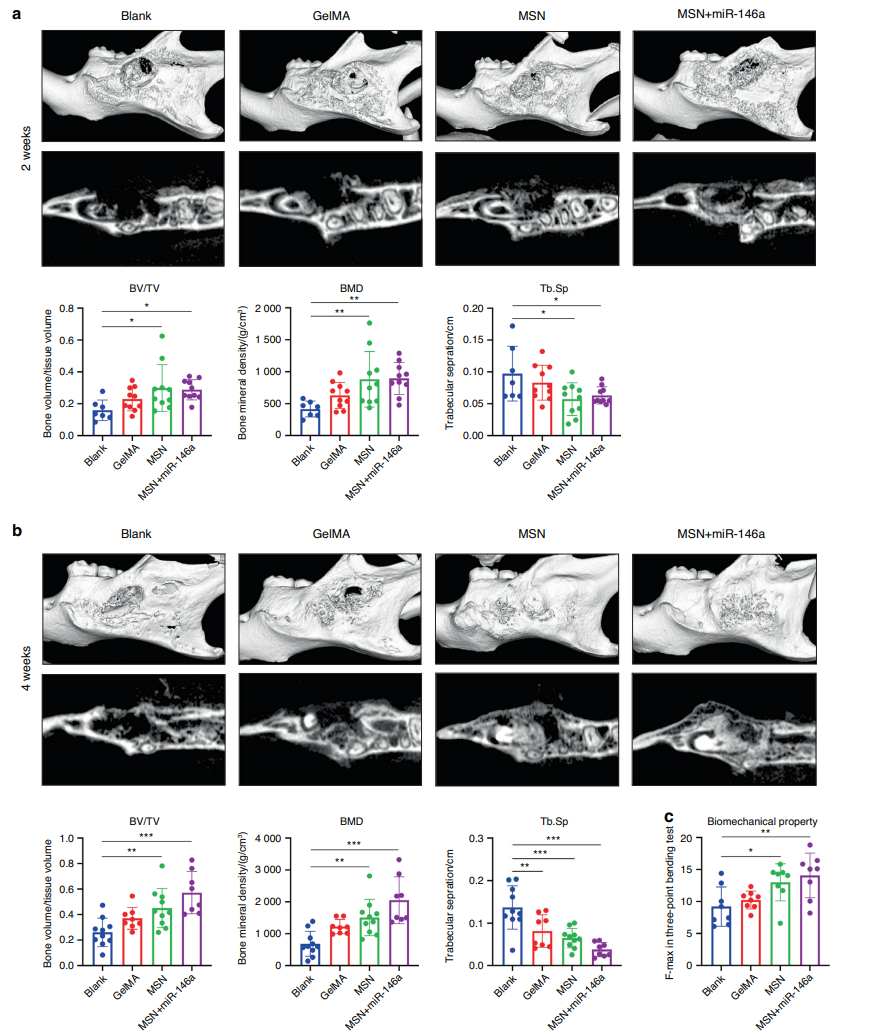
Figure 4. MSNs+miR-146a accelerated bone regeneration in a stimulated infected mouse mandibular defect model. a, b Micro-CT examinations of mouse mandible samples after 2 weeks (a) and 4 weeks (b) of healing following surgery with representative reconstructed volume and slice images and the mean BV/TV, BMD and Tb.Sp (n = 7–10). c Maximal force detected in the three-point bending test of trimmed mouse mandible samples (n = 8). *P < 0.05, **P < 0.01, ***P < 0.001. One-way ANOVA with Dunnett’s multiple comparisons to the blank group was used.
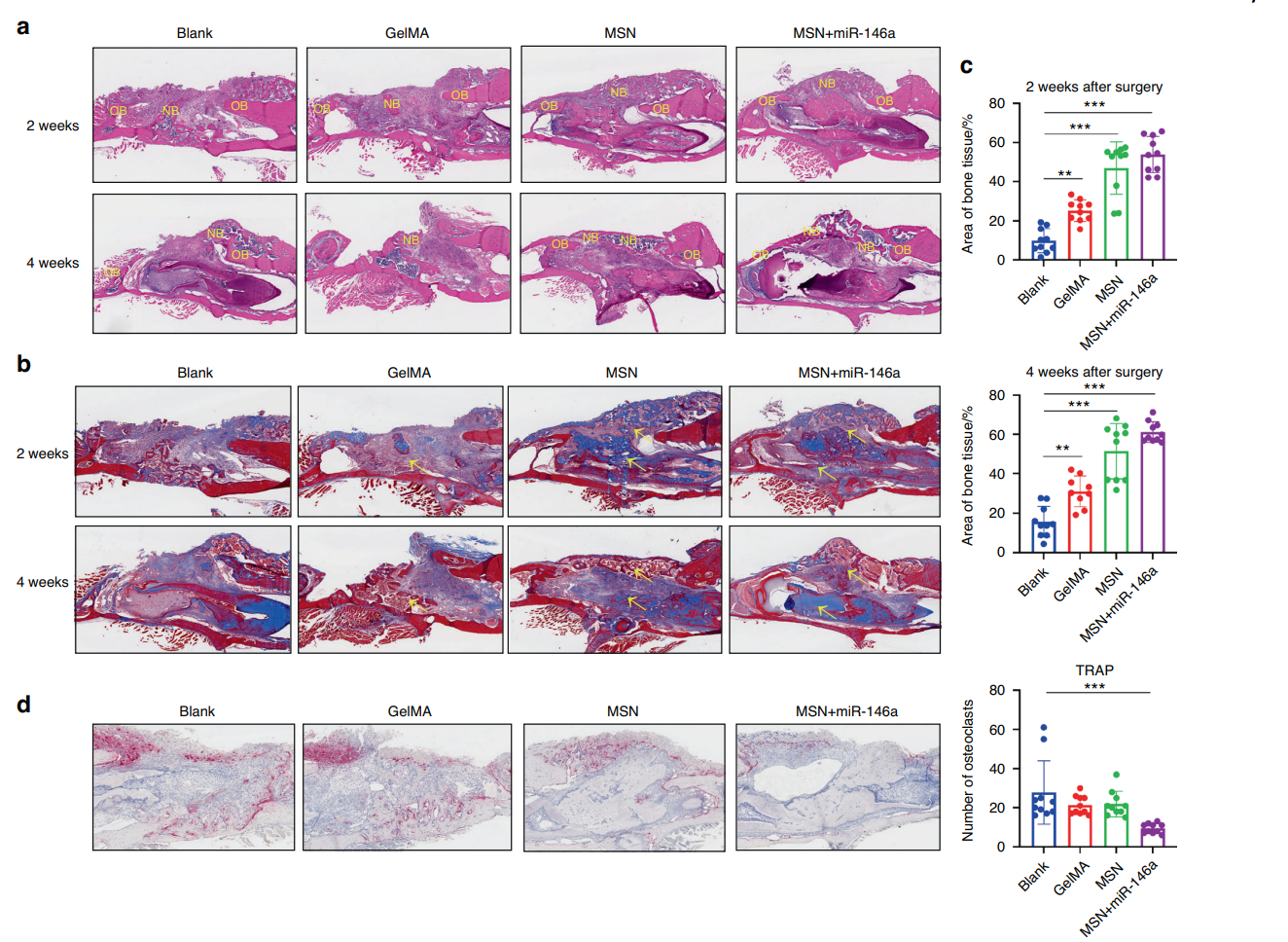
Figure 5. MSNs+miR-146a promoted bone regeneration but inhibited osteoclast formation. a HE staining of the mouse mandible samples after 2 and 4 weeks of healing. NB new bone, OB original bone. b Masson staining of the samples after 2 and 4 weeks of healing. Yellow arrows indicate blue-stained type I collagen of new bone. c Statistical analysis of the area proportion of bone tissues in Masson-stained slices of the samples after 2 and 4 weeks of healing (n = 9–10). d TRAP staining and quantitative analysis (n = 10) of the samples after 2 weeks of healing. *P < 0.05, **P < 0.01, ***P < 0.001 One-way ANOVA with Dunnett’s multiple comparisons to the blank group was used.
Novel Aspect
The in vivo study demonstrated the practical application of the MSN+miR-146a complex in a clinically relevant model, underscoring its efficacy in bone regeneration within an inflammatory context. This approach showcases an innovative strategy for addressing complex oral-maxillofacial defects that traditional methods often fail to resolve effectively.
Conclusion
The successful development of the microRNA-146a-loaded magnesium silicate nanospheres (MSNs) as a novel drug delivery system has been achieved through a comprehensive approach that integrates advanced nanotechnology with targeted therapeutic strategies. By optimizing the loading capacity of miR-146a onto MSNs and ensuring their biocompatibility, the researchers have created a delivery vehicle that not only enhances the localized release of miR-146a but also effectively addresses the challenges posed by inflammatory environments.
The highlights of this study include the demonstration of the MSN+miR-146a complex’s dual functionality: promoting osteogenic differentiation of human dental pulp stem cells (hDPSCs) while simultaneously modulating the inflammatory response of bone marrow-derived macrophages (BMMs). In vivo results further validated the efficacy of this biomaterial in enhancing bone regeneration in a stimulated infected mouse mandibular defect model. Overall, this innovative approach presents a significant advancement in the field of regenerative medicine, paving the way for more effective and targeted therapies for complex oral-maxillofacial bone defects.
Reference
Yang, Jiakang, et al. “MicroRNA-146a-loaded Magnesium Silicate Nanospheres Promote Bone Regeneration in an Inflammatory Microenvironment.” Bone Research, vol. 12, no. 2, 2024, doi:10.1038/s41413-023-00299-0.
