Editor: Nina
Scientists design and characterize functionalized iron oxide nanoparticles to enhance the loading and controlled release of doxorubicin, aiming to improve targeted cancer therapy while minimizing side effects.
Key Preview
- Research Question: How do differently functionalized iron oxide nanoparticles (IONs) impact the loading and controlled release of the anticancer drug doxorubicin?
- Research Design and Strategy: The study synthesized and characterized four types of IONs functionalized with cationic (polyethylenimine, PEI), anionic (polystyrenesulfonate, PSS), nonionic (dextran), and porous carbon to determine their drug loading capacities and release behaviors.
- Method: The IONs were characterized using X-ray diffraction, infrared spectroscopy, high-resolution transmission electron microscopy (HRTEM), scanning electron microscopy (SEM), and zeta potential measurements across different pH levels. Doxorubicin loading and desorption were measured at physiological and acidic pH.
- Key Results: The study found that PEI-modified IONs exhibited the highest doxorubicin loading capacity (65%), while PSS-modified IONs showed the most significant release (up to 30%) at pH 5.
- Significance of the Research: This research suggests that functionalized IONs could enhance the efficacy and safety of doxorubicin delivery in cancer therapy, potentially minimizing side effects associated with traditional chemotherapeutic methods.
Introduction
Cancer remains one of the leading causes of morbidity and mortality worldwide, with millions of new cases diagnosed each year. The disease is characterized by uncontrolled cell growth and proliferation, leading to the formation of tumors that can invade surrounding tissues and metastasize to distant organs. Among various treatment modalities, chemotherapy plays a crucial role in managing cancer, utilizing cytotoxic agents to target and eliminate rapidly dividing cancer cells. Doxorubicin (DOX), a widely used chemotherapeutic agent, has demonstrated significant efficacy against various malignancies, including breast cancer, leukemia, and sarcomas. However, its clinical use is severely limited by adverse side effects, including cardiotoxicity and damage to healthy tissues, which often lead to dose reduction and treatment discontinuation.
Traditional drug delivery methods primarily rely on systemic administration, where chemotherapeutic agents are administered intravenously or orally. This approach aims to saturate the bloodstream with the drug, allowing it to reach tumor sites through the circulatory system. While this method can be effective in delivering the drug, it is fraught with challenges. A major issue is the lack of specificity, resulting in the unintended exposure of healthy cells to cytotoxic agents. This non-selective action leads to systemic toxicity, causing severe side effects that can significantly impact the patient’s quality of life and limit the overall effectiveness of the treatment.
The challenges associated with conventional chemotherapy underscore the need for innovative drug delivery strategies that can enhance therapeutic efficacy while minimizing toxicity. One promising approach involves the use of nanotechnology, particularly functionalized iron oxide nanoparticles (IONs), as drug carriers. These nanoparticles can be engineered to provide targeted drug delivery, allowing for localized treatment of tumors while sparing healthy tissues. They can be modified with various surface coatings to improve biocompatibility, increase drug loading capacity, and enable controlled release in response to the tumor microenvironment. By harnessing the unique properties of nanoparticles, this innovative drug delivery strategy aims to overcome the limitations of traditional treatment, ultimately improving therapeutic outcomes for cancer patients.
Research Team and Aim
The research team for this study was led by Vladislav R. Khabibullin, who conducted the research in collaboration with co-authors Margarita R. Chetyrkina, Sergei I. Obydennyy, Sergey V. Maksimov, Gennady V. Stepanov, and Sergei N. Shtykov. This research was published on February 24, 2023, in the International Journal of Molecular Sciences, under the title “Study on Doxorubicin Loading on Differently Functionalized Iron Oxide Nanoparticles: Implications for Controlled Drug-Delivery Application.”
The aim of the research, as articulated by Khabibullin, was to enhance the delivery of doxorubicin through the innovative use of functionalized iron oxide nanoparticles, ultimately improving therapeutic outcomes in cancer treatment while reducing associated side effects. The study seeks to explore the loading capacities and release behaviors of these nanoparticles to address the critical challenges posed by traditional chemotherapy methods.
Experimental Process
Experiment 1: Synthesis and Characterization of Iron Oxide Nanoparticles (IONs)
Primary Technique:
The primary technique utilized for the synthesis of iron oxide nanoparticles (IONs) was a precipitation method under alkaline conditions. This method allows for the controlled formation of cubic magnetite (Fe3O4) particles.
Key Steps:
- Preparation of Alkaline Solution: Dissolve 3.86 g of sodium hydroxide (NaOH) in 400 mL of deionized water under a nitrogen atmosphere.
- Preparation of Iron(II) Solution: Dissolve 10.0 g of iron(II) sulfate heptahydrate (FeSO4·7H2O) in 100 mL of deionized water under nitrogen.
- Reaction Setup: Add the iron(II) solution to the heated alkaline solution at 80 °C while stirring at 2000 rpm.
- Aeration: Pass a current of air (1000 mL·min⁻¹) through the mixed solution for 2 hours, causing the solution to change color from light blue to black, indicating the formation of nanoparticles.
- Isolation: Isolate the resulting IONs by centrifugation at 6000 rpm for 10 minutes, wash several times with deionized water and ethanol, and disperse in propylene glycol.
Data Collection and Analysis:
The synthesized IONs were characterized using various techniques, including X-ray diffraction (XRD) for phase identification, and high-resolution transmission electron microscopy (HRTEM) for morphology and size analysis. The particle size distribution was determined by dynamic light scattering (DLS).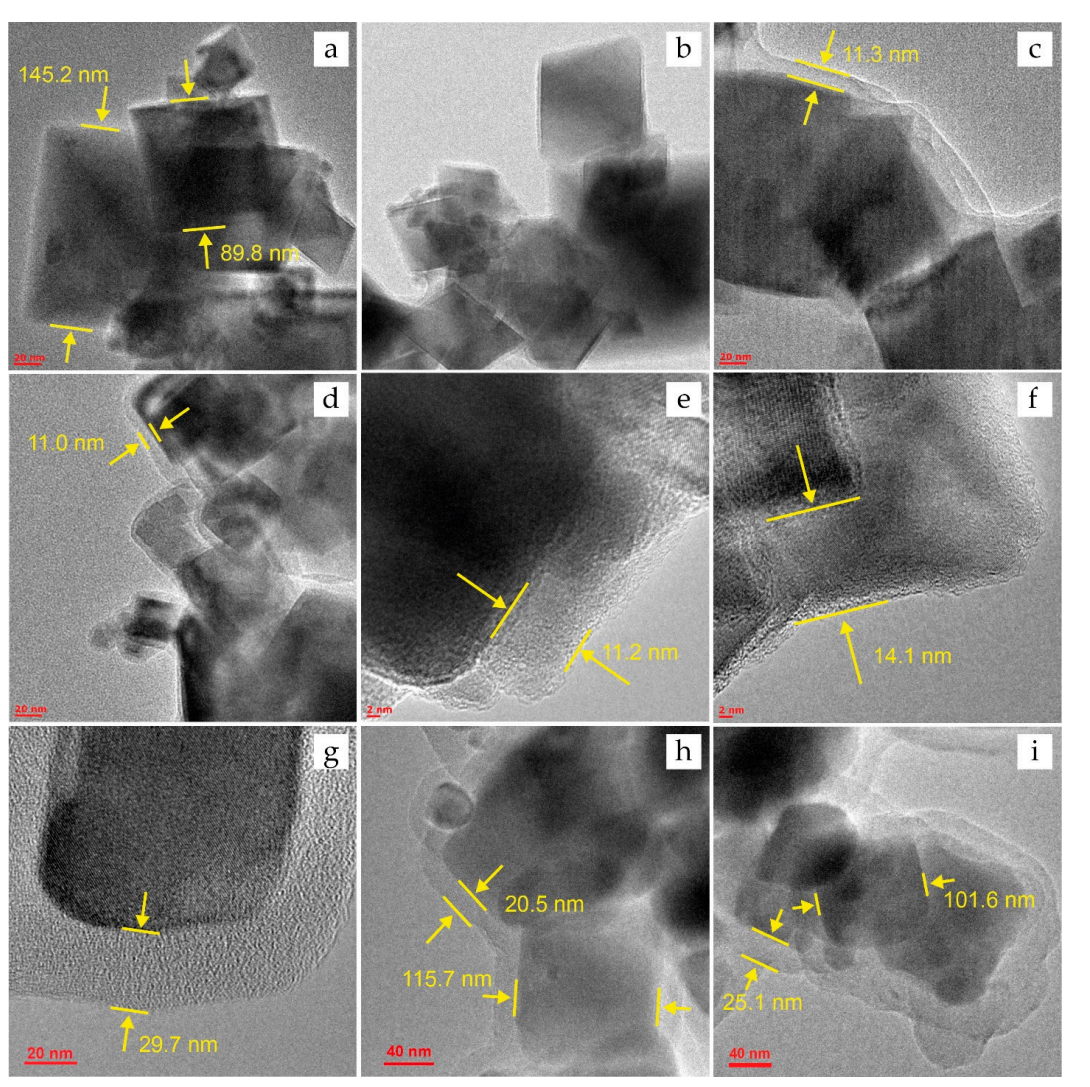
Figure 1. HRTEM images for the IONs before DOX sorption: (a)—Fe3O4, ©—Fe3O4@PSS, (e)— Fe3O4@PEI, and (g,h)—Fe3O4@Carb; and after sorption of DOX: (b)—Fe3O4@DOX, (d)— Fe3O4@PSS@DOX, (f)—Fe3O4@PEI@DOX, and (i)—Fe3O4@Carb@DOX.
Result:
The synthesis yielded cubic magnetite nanoparticles with an average size of 115 ± 35 nm, confirmed by HRTEM images. The XRD patterns matched standard data, confirming the successful synthesis of Fe3O4.
Novel Aspect:
This method allows for the straightforward and inexpensive synthesis of IONs with controlled size, which is advantageous over more complex synthesis routes commonly used in traditional nano-delivery systems.
Experiment 2: Functionalization of IONs
Primary Technique:
The main technique employed for functionalizing the IONs involved surface modification with cationic (polyethylenimine, PEI), anionic (polystyrenesulfonate, PSS), and nonionic (dextran) polymers.
Key Steps:
- Preparation of ION Suspension: Disperse synthesized IONs in deionized water to obtain a 100 µg·mL⁻¹ suspension.
- Addition of Functionalizing Agent: Mix the ION suspension with a functionalizing agent solution (e.g., PEI, PSS, or dextran) at a 1:10 ratio.
- Sonication: Sonicate the mixture for 10 minutes to promote uniform coating.
- Stirring: Stir the mixture for 24 hours at 50 rpm to ensure thorough interaction between IONs and the functionalizing agents.
- Washing and Isolation: Wash the modified particles several times with water and ethanol, and isolate by centrifugation at 4000 rpm.
Data Collection and Analysis:
The success of the functionalization was confirmed using Fourier-transform infrared spectroscopy (FTIR) to identify characteristic peaks corresponding to the functionalizing agents. Zeta potential measurements were taken to assess the surface charge and stability of the nanoparticles.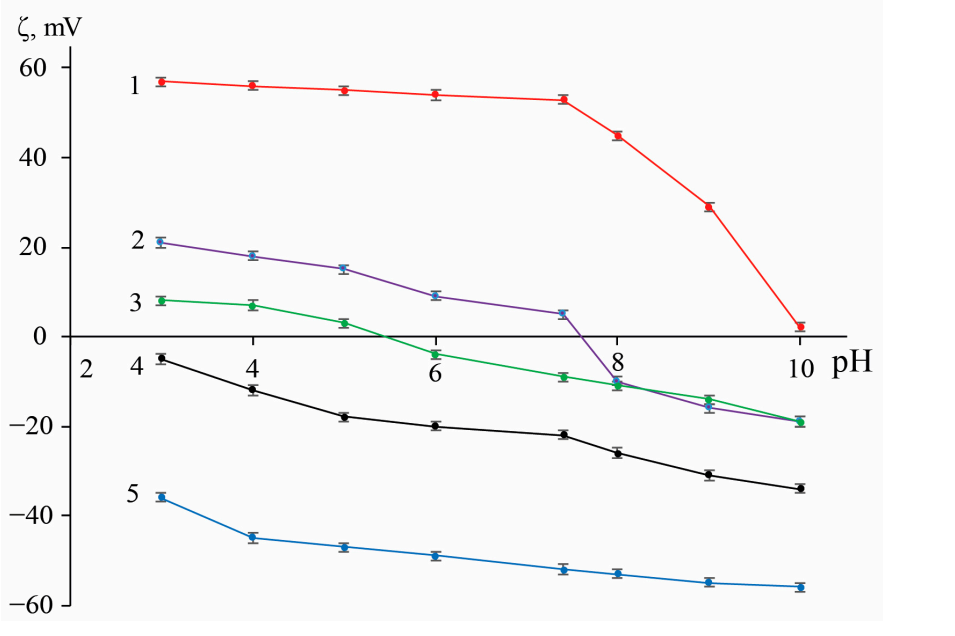
Figure 2. pH-responsive zeta-potential of original and functionalized IONs. Traces: (1)—Fe3O4@PSS, (2)—Fe3O4@Сarb, (3)—Fe3O4@Dex, (4)—Fe3O4, and (5)—Fe3O4@PEI (n = 3, p = 0.95)
Result:
FTIR spectra indicated successful functionalization with characteristic peaks corresponding to the functional groups of the polymers used. Zeta potential measurements showed that the modifications significantly improved the colloidal stability of the nanoparticles.
Novel Aspect:
The introduction of multiple types of surface modifiers allows for tailored interactions with the drug and improved biocompatibility. This multifunctional approach enhances the efficacy of drug delivery compared to traditional single-modifier systems.
Experiment 3: Doxorubicin Loading
Primary Technique:
The primary method for assessing doxorubicin (DOX) loading onto functionalized IONs was an adsorption study conducted in phosphate-buffered saline (PBS).
Key Steps:
- Preparation of DOX Solution: Prepare a 100 µg·mL⁻¹ solution of doxorubicin in PBS (pH 7.4).
- Sorption Process: Add 5 mL of the DOX solution to 5 mL of the ION suspension (at a concentration of 100 µg·mL⁻¹) in a polypropylene tube.
- Incubation: Stir the mixture for predetermined time intervals (e.g., 2, 5, 10, 15, 30, and 45 minutes) at 20 rpm.
- Separation: After incubation, use an external magnetic field to separate the IONs and collect the supernatant for analysis.
- Concentration Measurement: Assess the concentration of unbound DOX in the supernatant spectrophotometrically.
Data Collection and Analysis:
Data on the concentration of unbound DOX was collected at each time point to construct a sorption isotherm. The degree of loading was calculated using the difference between the initial and residual concentrations of DOX.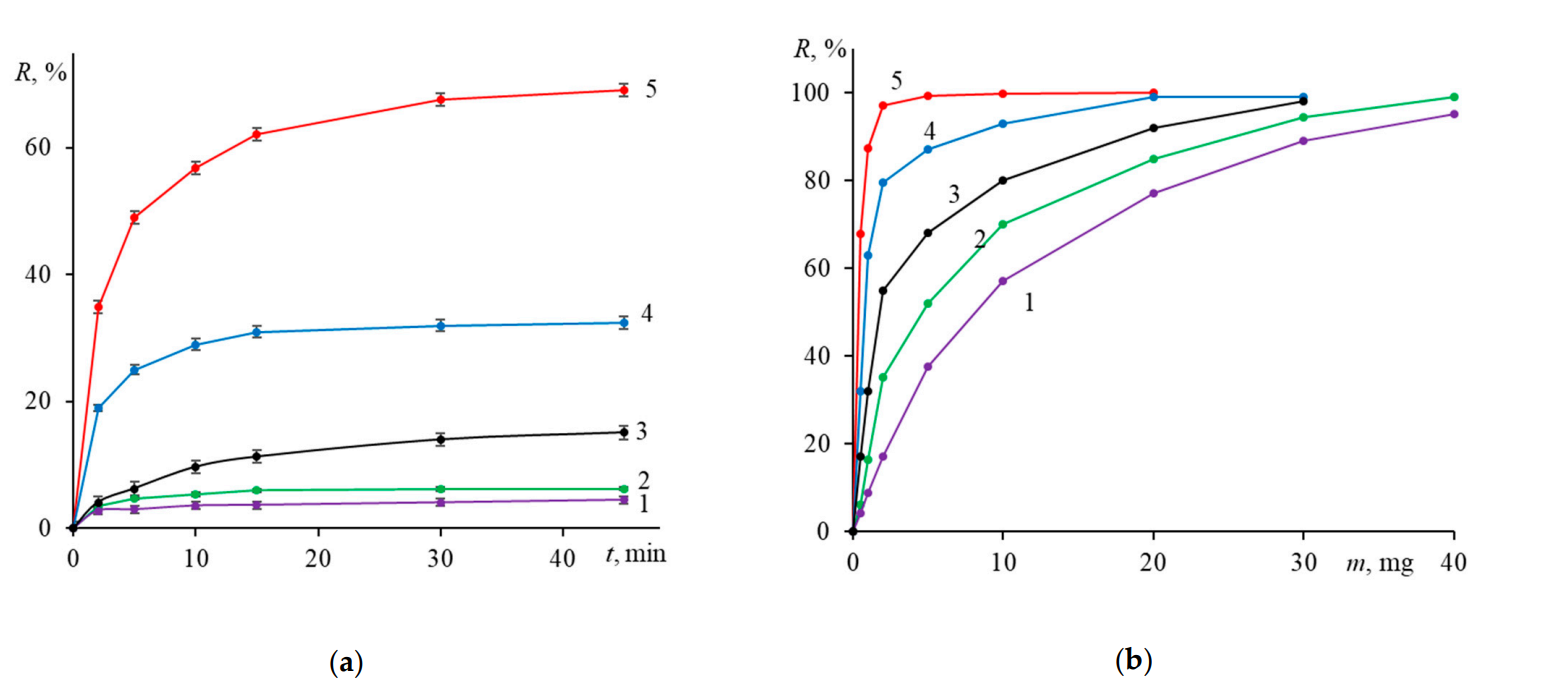
Figure 3. Time- and sorbent mass-dependent sorption of doxorubicin on various IONs. Conditions: (a) m0 = mS = 0.5 mg and (b) mS = 0.5 mg; time 45 min, pH 7.4, 25 °C. Traces: (1)—Fe3O4, (2)— Fe3O4@Dex, (3)—Fe3O4@Сarb, (4)—Fe3O4@PSS, and (5)—Fe3O4@PEI. (n = 3, p = 0.95).
Result:
The PEI-modified IONs achieved the highest loading capacity of 65%, with significant adsorption occurring within the first 15 minutes, as demonstrated by the rapid decrease in supernatant DOX concentration.
Novel Aspect:
This study demonstrates a highly efficient loading mechanism that leverages the strong electrostatic interactions between the positively charged PEI and the negatively charged DOX, providing a more effective drug delivery system compared to conventional methods that typically rely on passive diffusion.
Experiment 4: Doxorubicin Release Studies
Primary Technique:
The main technique used to evaluate the release kinetics of doxorubicin from the functionalized IONs was a controlled release study simulating physiological and acidic environments.
Key Steps:
- Preparation of Release Medium: Prepare two buffer solutions: one at pH 7.4 (physiological) and another at pH 5.0 (acidic).
- Release Experiment Setup: Place 5 mg of the DOX-loaded nanoparticles in 50 mL of each buffer solution.
- Stirring: Stir the solutions at 20 rpm for predetermined time intervals.
- Sampling: At set time points (5, 10, 15, 30, 60, 120, 360, and 1440 minutes), withdraw 5 mL of the solution, and separate the nanoparticles using a magnet.
- Concentration Measurement: Measure the concentration of released DOX in the supernatant spectrophotometrically.
Data Collection and Analysis:
The cumulative release of doxorubicin was calculated at each time point, and the data were analyzed using release kinetics models to determine the mechanism of drug release.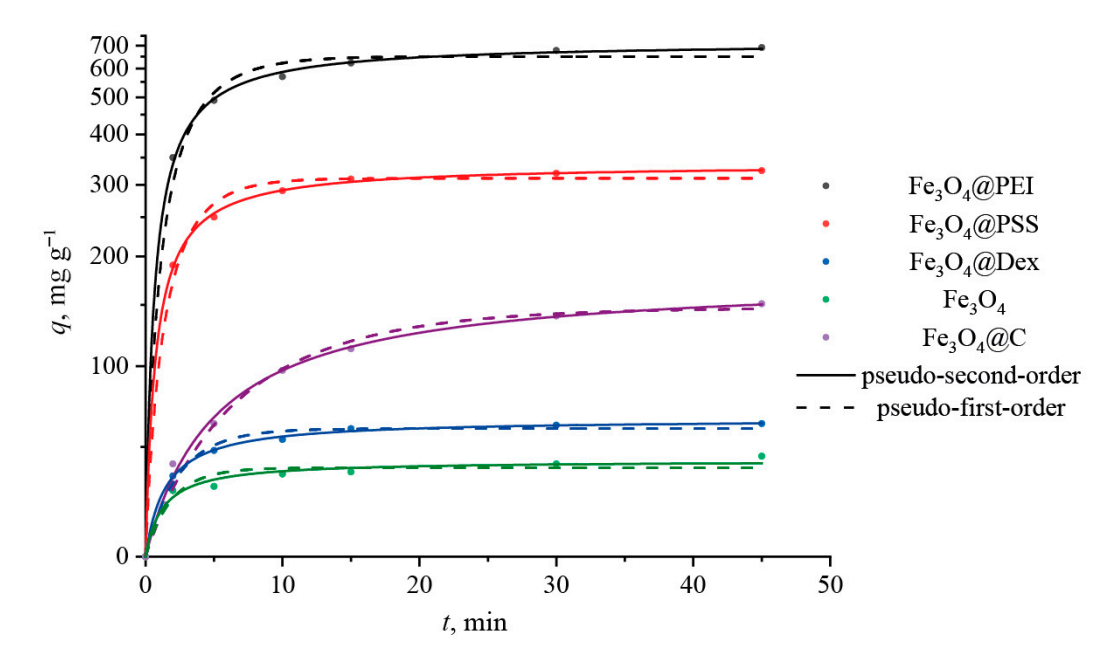
Figure 4. Adsorption kinetic curves of DOX onto different magnetite nanoparticles.
Result:
At pH 5.0, a maximum release of approximately 30% was observed for the PSS-modified IONs within the first 30 minutes, while the release at pH 7.4 was less than 7%.
Novel Aspect:
The study highlights the potential for targeted drug release in response to the acidic tumor microenvironment, significantly enhancing therapeutic efficacy while minimizing systemic toxicity, a notable improvement over traditional release systems that lack this level of environmental responsiveness.
Experiment 5: Cytotoxicity and Biocompatibility Assessment
Primary Technique:
The primary technique for evaluating cytotoxicity involved the MTT assay, which assesses cell viability in the presence of functionalized IONs.
Key Steps:
- Cell Culture: Culture Neuro2A (N2A) cell lines in DMEM media supplemented with FBS and antibiotics.
- Cell Seeding: Seed cells in 96-well plates at a density of 10,000 cells per well and incubate for 24 hours at 37 °C.
- Addition of Nanoparticles: Treat cells with varying concentrations of functionalized IONs (1, 5, 10, 50, and 100 µg·L⁻¹).
- MTT Assay: After 24 hours of incubation, add MTT solution to each well and incubate for additional hours.
- Measurement: Measure the absorbance at 570 nm using a spectrophotometer.
Data Collection and Analysis:
Data were collected on cell viability relative to control groups (without nanoparticles). Statistical analyses (e.g., ANOVA) were performed to assess the significance of differences observed.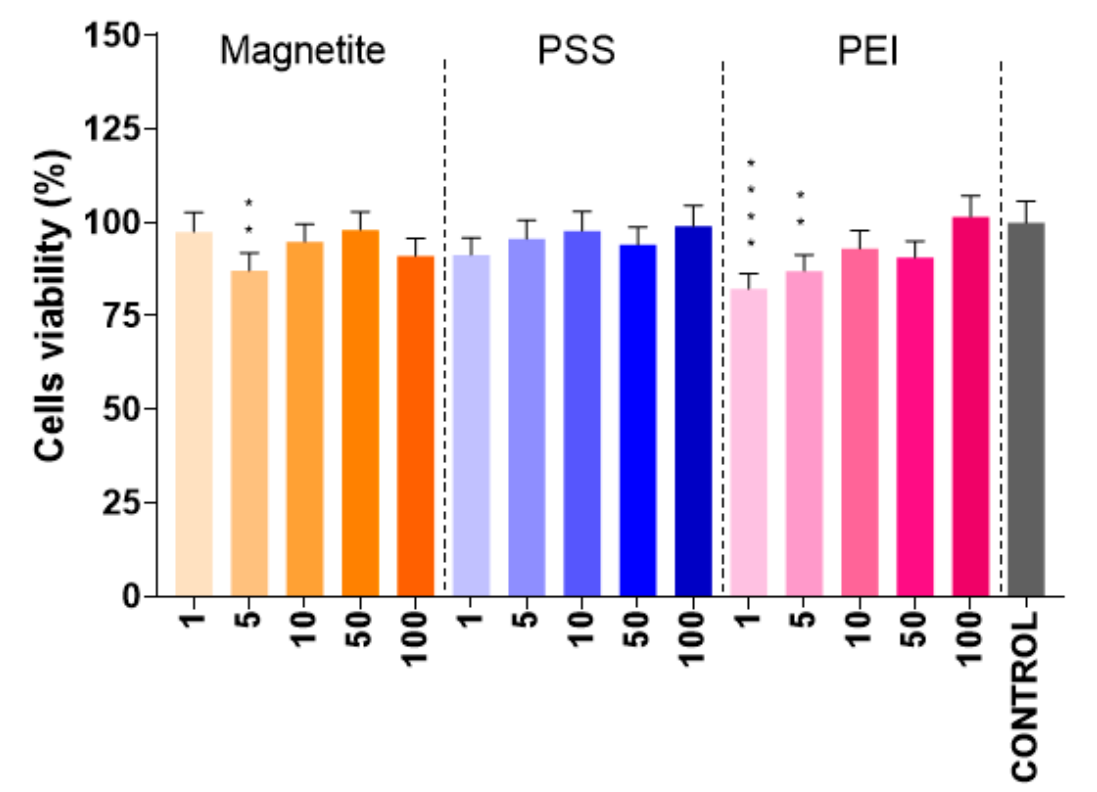
Figure 5. Results of the cell viability MTT test for N2A cells incubated with parent and modified IONs. The abscissa numbers represent concentration of nanoparticles (in µg·L−1); the p values (<0.01 and < 0.001) are marked, respectively, with,two, and four asterisks.
Result:
The presence of PEI- and PSS-modified IONs did not statistically reduce cell viability, with survival rates exceeding 75-80%.
Novel Aspect:
This experiment demonstrated the biocompatibility of the modified nanoparticles, an important consideration for in vivo applications, and showcased their potential for safe therapeutic use compared to traditional drug delivery systems that may be more cytotoxic.
Conclusion
The successful development of the drug delivery system presented in this study is achieved through a systematic approach that combines the synthesis of functionalized iron oxide nanoparticles (IONs) with the optimization of doxorubicin loading and controlled release mechanisms. By employing a precipitation method for synthesizing IONs and subsequently modifying their surfaces with various polymers, the researchers were able to enhance drug loading capacities while ensuring biocompatibility and stability of the nanoparticles in aqueous environments.
A highlight of the study is the significant finding that PEI-modified IONs exhibited the highest doxorubicin loading capacity of 65%, while PSS-modified IONs demonstrated the most effective drug release profile, achieving approximately 30% release at pH 5.0, which simulates the acidic tumor microenvironment. This innovative approach not only addresses the limitations of traditional chemotherapy by minimizing systemic toxicity but also enhances the therapeutic efficacy of doxorubicin delivery. The results indicate that functionalized IONs have the potential to serve as an efficient and targeted drug delivery platform, paving the way for improved outcomes in cancer treatment.
Reference
Khabibullin, Vladislav R., Margarita R. Chetyrkina, Sergei I. Obydennyy, Sergey V. Maksimov, Gennady V. Stepanov, and Sergei N. Shtykov. “Study on Doxorubicin Loading on Differently Functionalized Iron Oxide Nanoparticles: Implications for Controlled Drug-Delivery Application.” International Journal of Molecular Sciences, vol. 24, no. 4480, 2023, https://doi.org/10.3390/ijms24054480.
