Editor: Tiffany
New research reveals that polydopamine-coated liposomes significantly improve the delivery and effectiveness of methylene blue in photodynamic therapy, offering a promising advancement in cancer treatment.
Key Highlights
- Research Question:
The study aimed to assess the efficacy of polydopamine-coated liposomes as a delivery system for methylene blue in photodynamic therapy, specifically evaluating their effects on cancer cells in both 2D and 3D cultures. - Research Difficulties:
The primary challenges included overcoming the limitations of existing photosensitizers, such as low solubility and poor cellular uptake, which hinder their effectiveness in clinical applications. - Key Findings:
The research demonstrated that polydopamine-coated liposomes (lipoPDA@MB) exhibited higher photodynamic potency compared to free methylene blue, primarily due to enhanced cellular uptake and reactive oxygen species production. - Innovative Aspects:
This study introduces a novel drug delivery system that utilizes polydopamine to coat liposomes, allowing for more effective adsorption of methylene blue and improving its stability and release profile. - Importance of the Study:
The findings underscore the potential of lipoPDA@MB as an efficient third-generation photosensitizer in photodynamic therapy, paving the way for more effective cancer treatments with reduced side effects.
Mechanisms of Photodynamic Therapy in Cancer Treatment
Cancer remains one of the toughest diseases to beat, but a treatment called Photodynamic Therapy (PDT) offers a ray of hope. PDT uses special drugs called photosensitizers, along with light and oxygen, to attack cancer cells. When light hits these drugs, they produce harmful substances known as reactive oxygen species (ROS), which damage and kill cancer cells. What makes PDT special is its ability to target tumors while leaving healthy tissues mostly unharmed. According to research, this therapy can trigger different ways for cancer cells to die, such as apoptosis (a controlled cell death), necrosis (a messier cell death), or autophagy (where cells break themselves down).
One photosensitizer, methylene blue (MB), stands out because it can generate both ROS and another powerful molecule called singlet oxygen. This makes it effective even in tumors with low oxygen levels, a common challenge in cancer treatment. However, MB isn’t perfect—it doesn’t dissolve well in water and struggles to get inside cancer cells on its own.
PDT has come a long way. Early drugs, known as first-generation photosensitizers like hematoporphyrin derivatives, had drawbacks such as causing skin sensitivity to light and not penetrating deep into tissues. Second-generation photosensitizers improved on these issues, but they still weren’t ideal. Now, third-generation photosensitizers, which use advanced systems like nanoparticles, aim to deliver drugs more effectively and cut down on side effects. Even so, getting these drugs to the right spot in the body without causing harm remains a hurdle.
Objectives of the Study
Scientists set out to solve MB’s delivery problems. Their big question: Could they find a better way to get MB into cancer cells and make it work more effectively in PDT? MB has great potential, but its low solubility and poor uptake by cells hold it back. The goal of this study was to create a new delivery system using polydopamine-coated liposomes (lipoPDA@MB)—tiny fat-based carriers coated with a sticky polymer—to carry MB, protect it, and boost its cancer-killing power.
The research team included experts like Marzia Bruna Gariboldi from the University of Insubria, Vincenzo De Leo from the University of Bari Aldo Moro, and others from the Italian National Research Council. Their findings were published in the International Journal of Molecular Sciences in March 2024.
Experimental Design and Key Findings
I. Experimental Process (Outline)
- Preparation of uncoated small unilamellar liposomes using the Micelle-To-Vesicle Transition (MVT) method.
- Coating of liposomes with polydopamine (PDA) at pH 8.0.
- Characterization of physical properties of uncoated and PDA-coated liposomes using Dynamic Light Scattering (DLS) and ζ-potential analysis.
- Preparation of methylene blue (MB)-loaded PDA-coated liposomes (lipoPDA@MB) by incubating lipoPDA with MB solution.
- Purification of lipoPDA@MB vesicles from unbound MB using size exclusion chromatography (SEC).
- Assessment of in vitro release of MB from lipoPDA@MB vesicles.
- Evaluation of photostability of MB and lipoPDA@MB under irradiation.
- Evaluation of photodynamic activity using the MTT assay on various cancer cell lines.
- Assessment of cellular uptake of lipoPDA@MB and MB using flow cytometry.
- Analysis of cell death mechanisms induced by lipoPDA@MB and MB.
II. Key Experiments
1. Procedure: In Vitro Release Assay
- The in vitro cumulative release of MB from lipoPDA@MB vesicles was assessed in phosphate-buffered saline (PBS) at physiological pH using a dialysis-based method.
Result:
- The release profile exhibited a rapid release of MB within the first 4 hours, followed by a slower release phase, ultimately reaching a plateau of approximately 45% MB release by 7 hours, with no further significant changes observed up to 24 hours.
Finding:
- The results indicated that the slow release from the PDA-coated liposomes could be beneficial for maintaining drug concentration until reaching the tumor site.
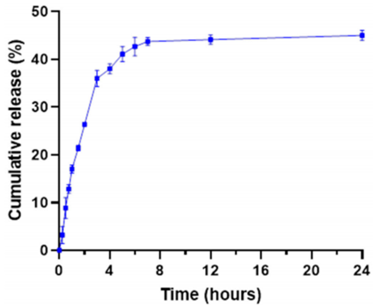
Figure 1. Cumulative in vitro release of MB from lipoPDA@MB vesicles in PBS 1X, pH 7.4, demonstrating a fast initial release followed by a slower release phase.
2. Procedure: Photostability Assessment
- The photodegradation of MB and lipoPDA@MB was evaluated by measuring the decrease in absorbance intensity during 90 minutes of irradiation with a white tungsten halogen light.
Result:
- The photostability analysis revealed that lipoPDA@MB exhibited significantly less photobleaching compared to free MB over the same period.
Finding:
- This suggests that the PDA coating protects MB from photodegradation, enhancing its stability during photodynamic therapy.
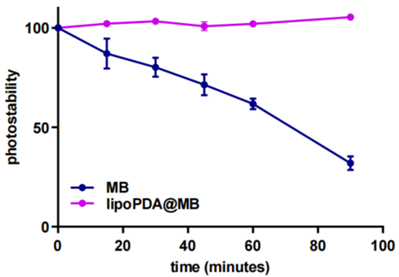
Figure 2. Photostability of MB and lipoPDA@MB during 90 min irradiation with a halogen light, indicating reduced photobleaching of lipoPDA@MB compared to free MB.
3. Procedure: MTT Assay for Cell Viability
- The photodynamic activity of MB and lipoPDA@MB was tested on HCT116, HT29, MCF7, and MDA-MB231 cell lines. Cells were treated with increasing concentrations of both formulations, followed by irradiation and subsequent incubation.
Result:
- Dose-response curves indicated that lipoPDA@MB demonstrated significantly lower IC50 values compared to free MB across all cell lines, indicating enhanced potency.
Finding:
- The enhanced toxicity of lipoPDA@MB underscores its potential as an effective photosensitizer in photodynamic therapy.
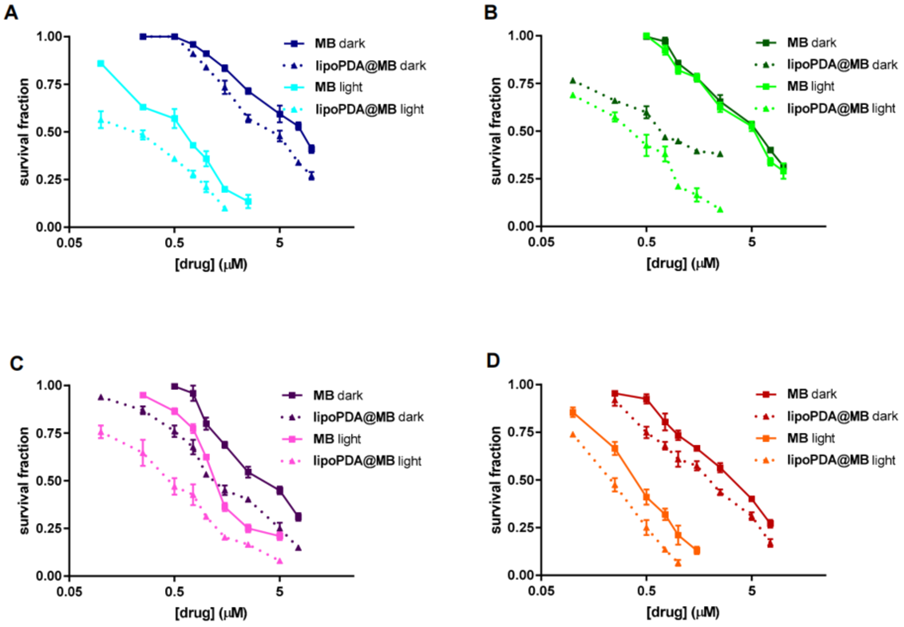
Figure 3. Dose-response curves obtained following 24 h treatment of HCT116, HT29, MCF7, and MDA-MB231 cells with MB and lipoPDA@MB, showing enhanced potency of lipoPDA@MB.
4. Procedure: Cellular Uptake Evaluation
- The uptake of lipoPDA@MB and MB was assessed through flow cytometry using fluorescently labeled formulations.
Result:
- Higher fluorescence levels were observed in lipoPDA@MB-treated cells compared to those treated with free MB, indicating increased cellular uptake of lipoPDA@MB.
Finding:
- This enhanced uptake likely contributes to the improved photodynamic efficacy observed with lipoPDA@MB.
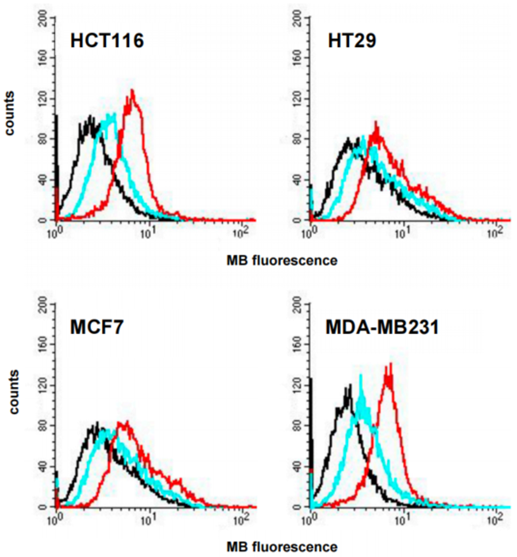
Figure 4. Intracellular uptake of MB and lipoPDA@MB in various cancer cell lines, demonstrating higher fluorescence levels in lipoPDA@MB-treated cells compared to those treated with free MB.
Implications and Future Directions
This study unveils a game-changer for PDT: polydopamine-coated liposomes that deliver methylene blue more effectively than ever. These tiny carriers, just 63 nm wide, protect MB from light damage, release it slowly (45% over 24 hours), and help it sneak into cancer cells. Once inside, they boost ROS production and trigger apoptosis, wiping out cancer cells more efficiently than MB alone. The results were especially striking in tough cancers like triple-negative breast cancer (MDA-MB-231 cells).
The innovation here is a smarter delivery system that overcomes MB’s natural weaknesses. The significance? This could lead to better, safer cancer treatments, offering new hope for patients facing hard-to-treat tumors. As research moves forward, this approach might light the way to more precise and powerful therapies.
Reference:
De Leo, Vincenzo, et al. “Polydopamine-coated liposomes for methylene blue delivery in anticancer photodynamic therapy: effects in 2D and 3D cellular models.” International Journal of Molecular Sciences 25.6 (2024): 3392.
