Editor: Tiffany
Key Highlights
- Research Question:
What is the anti-cancer potency of copper-doped carbon quantum dots (Cu-CDs) against breast cancer progression, and what mechanisms underlie their effects on malignant cell behaviors? - Research Difficulties:
Breast cancer’s high mortality, lack of early symptoms, drug resistance, and toxic side effects of current treatments, such as chemotherapy and hormone therapy, complicate effective management. - Key Findings:
Cu-CDs reduced viability, proliferation, migration, invasion, adhesion, and clonogenicity of MDA-MB-231 cells, induced redox imbalance, cell cycle arrest, and apoptosis via mitochondrial dysfunction, and modulated the MAPK signaling pathway. - Innovative Aspects:
The study uses Cu-CDs, synthesized via a one-step hydrothermal method, to target breast cancer cells selectively while maintaining biocompatibility, addressing limitations of conventional therapies. - Importance of the Study:
The findings confirm the biosafety and efficacy of Cu-CDs, supporting their potential as a novel nanomaterial-based therapy for breast cancer, with implications for future clinical investigations.
Breast Cancer Challenges and Treatment Limitations
Breast cancer remains a global health crisis, striking millions of women each year and ranking among the most deadly cancers. According to the World Health Organization, it is one of the most common malignancies worldwide, with high mortality rates due in part to its often-silent progression. In its early stages, breast cancer typically presents no noticeable symptoms, leading to delayed diagnoses that worsen patient outcomes. When symptoms do emerge—such as lumps in the breast, changes in breast shape or size, skin dimpling, or nipple discharge—the disease may have already advanced, making treatment more challenging.
Diagnosing and treating breast cancer is fraught with difficulties. The disease’s complexity, with multiple subtypes like triple-negative breast cancer (e.g., MDA-MB-231 cells), means no single approach works for all patients. Current treatments include chemotherapy, hormone therapy, targeted therapies, and epigenetic drugs. While these have saved countless lives, they come with significant drawbacks. Chemotherapy, for instance, often causes toxic side effects like nausea, hair loss, and weakened immunity. Hormone and targeted therapies can lose effectiveness as cancer cells develop resistance. Even mastectomy, a drastic surgical option, fails to guarantee success, with recurrence and metastasis—cancer spreading to other parts of the body—remaining persistent threats. These limitations highlight an urgent need for new, innovative solutions to combat this devastating disease.
Investigating Cu-CDs as a Novel Breast Cancer Therapy
The stubborn challenges of breast cancer, particularly its tendency to metastasize and recur, have driven scientists to explore cutting-edge alternatives. A recent study from the Affiliated Hospital of Inner Mongolia Medical University, led by researcher Gang Liu, tackles this problem head-on by investigating a novel nanomaterial: copper-doped carbon quantum dots (Cu-CDs). Published in the International Journal of Nanomedicine in 2024, the study aims to evaluate the anti-cancer potency of Cu-CDs against breast cancer progression, specifically targeting the aggressive MDA-MB-231 cell line.
The research team set out with clear objectives: to assess how Cu-CDs affect key cancer cell behaviors—such as viability, proliferation, migration, invasion, adhesion, and colony formation—and to uncover the mechanisms behind these effects. By focusing on mitochondrial function, oxidative stress, and programmed cell death (apoptosis), along with analyzing genetic changes via transcriptomics, the study seeks to offer a fresh perspective on fighting breast cancer. The hope is that Cu-CDs could address the shortcomings of existing treatments, offering a safer, more effective option.
Experimental Approach and Anti-Cancer Outcomes
1. Experimental Process Outline:**
- Synthesis of copper-doped carbon quantum dots (Cu-CDs) using a one-step hydrothermal method.
- Characterization of Cu-CDs through transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), and Fourier transform infrared spectroscopy (FTIR).
- In vivo biocompatibility assessment of Cu-CDs in C57/BL6 mice.
- In vitro biocompatibility evaluation of Cu-CDs on normal cell lines: NRK52E, TM3, and MacT.
- Cytotoxicity effect assay of Cu-CDs on MDA-MB-231 breast cancer cells using the MTT assay.
- EdU staining assay to evaluate cell proliferation inhibition.
- Wound healing assay to assess cell migration capacity.
- Transwell invasion assay to evaluate the invasion ability of MDA-MB-231 cells.
- Cell adhesion assay to determine the adhesion capacity of MDA-MB-231 cells.
- Clonogenicity assay to assess the ability of MDA-MB-231 cells to form colonies.
- Flow cytometry to analyze cell cycle distribution.
- MitoTracker and JC-1 staining to evaluate mitochondrial function and membrane potential.
- Assessment of reactive oxygen species (ROS) production using DCFH-DA staining.
- Measurement of oxidative stress markers: Glutathione (GSH), superoxide dismutase (SOD), and malondialdehyde (MDA).
- Immunofluorescence staining for DNA damage (γH2A) and apoptosis assays (Annexin V staining).
- RNA sequencing (RNA-Seq) for transcriptomic analysis of gene expression changes.
2. Key Experiments
Experiment 1: Cytotoxicity Effect Assay (MTT Assay)
Procedure: MDA-MB-231 cells were seeded in 96-well plates and treated with varying concentrations of Cu-CDs (20, 40, 80, and 160 μg/mL) for 24 hours. Cell viability was assessed using the MTT assay.
Result: The viability of MDA-MB-231 cells significantly decreased with increasing concentrations of Cu-CDs, with a viability of 78.53% at 20 μg/mL, 66.03% at 40 μg/mL, 45.90% at 80 μg/mL, and 31.97% at 160 μg/mL.
Finding: Cu-CDs exhibit a dose-dependent cytotoxic effect on MDA-MB-231 breast cancer cells, indicating their potential as therapeutic agents.
Experiment 2: EdU Staining Assay
Procedure: MDA-MB-231 cells were treated with Cu-CDs and subsequently stained with EdU for 2 hours. The cells were then processed for imaging and analyzed for EdU incorporation to assess proliferation.
Result: The EdU staining intensity was significantly lower in the Cu-CDs treated group compared to the control group, indicating reduced proliferation.
Finding: Cu-CDs effectively inhibit the proliferation of MDA-MB-231 cells, further supporting their anti-cancer properties.
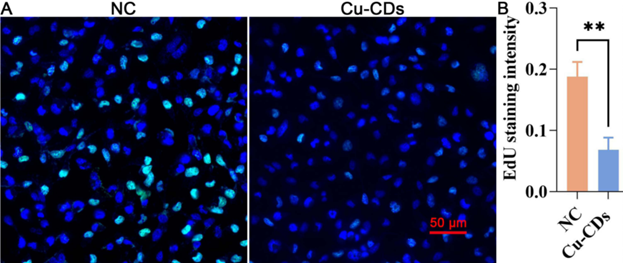
Figure 1. Representative EdU staining images and relative EdU staining intensity, showing significantly reduced proliferation in MDA-MB-231 cells treated with Cu-CDs compared to control.
Experiment 3: Wound Healing Assay
Procedure: A wound was created in confluent MDA-MB-231 cell monolayers using a pipette tip. Cells were then treated with Cu-CDs, and the closure of the wound was monitored over 24 hours.
Result: The wound healing capacity was significantly inhibited in the Cu-CDs treated group compared to the control group, with less closure observed.
Finding: Cu-CDs significantly impair the migratory ability of MDA-MB-231 cells, indicating a potential mechanism by which they inhibit cancer progression.
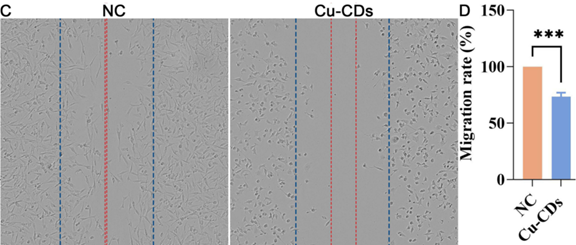
Figure 2. Representative results of the wound healing assay and quantitative analysis, indicating significant inhibition of wound closure in MDA-MB-231 cells treated with Cu-CDs.
Experiment 4: RNA Sequencing (RNA-Seq)
Procedure: Total RNA was extracted from MDA-MB-231 cells treated with Cu-CDs and analyzed for transcriptomic changes using RNA-Seq. Differentially expressed genes (DEGs) were identified and subjected to functional enrichment analysis.
Result: A total of 2065 genes were identified as differentially expressed, with 513 upregulated and 1552 downregulated in the Cu-CDs treated group.
Finding: The DEGs were significantly enriched in pathways related to the MAPK signaling pathway, indicating that Cu-CDs may exert their effects by modulating this critical pathway in breast cancer progression.
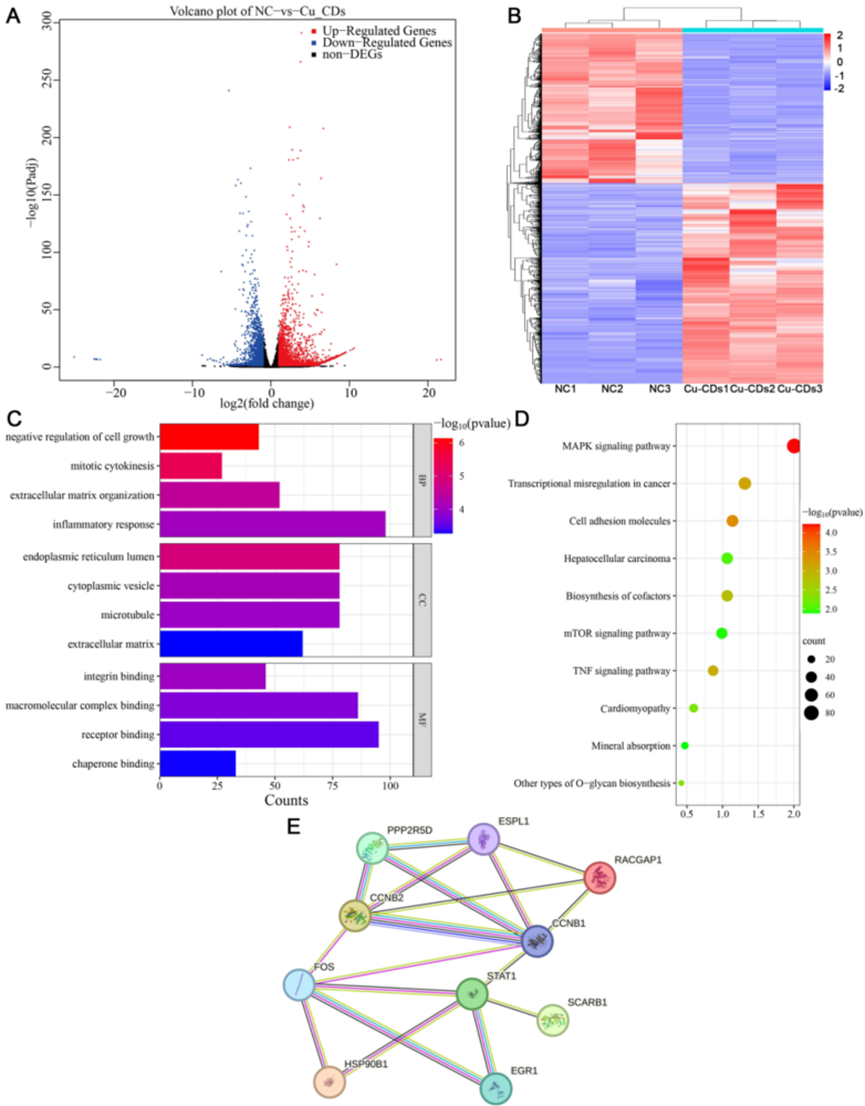
Figure 3. Volcano plot and heat map of differentially expressed genes (DEGs), GO analysis, KEGG pathway enrichment analysis, and protein-protein interaction (PPI) network analysis, illustrating the transcriptomic changes and potential regulatory pathways affected by Cu-CDs treatment in MDA-MB-231 cells.
Implications for Breast Cancer Treatment
This study unveils copper-doped carbon quantum dots as a promising weapon against breast cancer. By blending nanotechnology with cancer therapy, the research offers a novel solution to the limitations of conventional treatments like chemotherapy and surgery. The key findings—Cu-CDs’ ability to suppress cancer cell viability, halt proliferation, block metastasis, and trigger apoptosis while remaining safe for healthy tissues—suggest a future where breast cancer treatment could be both more effective and less harmful.
Reference:
Wang, Mengqi, et al. “Anti-cancer potency of copper-doped carbon quantum dots against breast cancer progression.” International Journal of Nanomedicine (2024): 1985-2004.
