Editor: Tiffany
A recent study demonstrates that delivering mRNAs encoding synaptic proteins via engineered extracellular vesicles can improve synaptic function and cognitive performance in a mouse model of Alzheimer’s disease, offering a potential new therapeutic approach for addressing synaptic dysfunction in AD.
Key Highlights
- Research Question:
Can increasing synaptic protein levels through mRNA delivery via extracellular vesicles improve cognitive function in Alzheimer’s disease? - Research Difficulties:
Delivering therapeutic agents across the blood-brain barrier to target the central nervous system and ensuring stable, effective expression of synaptic proteins in the brain. - Key Findings:
- Synaptic protein levels in neuronal-derived EVs were lower in AD patients and correlated with brain atrophy.
- In the 5xFAD mouse model, EV-mediated delivery of GAP43 and SNAP25 mRNAs increased synaptic protein expression, enhanced dendritic density, and improved cognitive performance.
- Innovative Aspects:
The use of engineered EVs to deliver mRNAs encoding synaptic proteins directly to the brain, bypassing the blood-brain barrier, and the first application of this approach in an AD mouse model. - Importance of the Study:
This research provides evidence that targeting synaptic dysfunction through mRNA delivery via EVs could be a viable therapeutic strategy for AD, addressing a core pathological feature of the disease.
Alzheimer’s Disease and Synaptic Dysfunction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder affecting approximately 10-30% of individuals over 65 years old, with a notable global prevalence. It is defined by cognitive decline, memory loss, and behavioral changes, such as confusion, language difficulties, and reduced daily functioning.
Existing AD treatments, including cholinesterase inhibitors and NMDA receptor antagonists, offer symptomatic relief but do not target the underlying mechanisms of the disease. Synaptic dysfunction is a central feature of AD pathology, underscoring the need for therapies addressing its root causes.
Targeting Synaptic Proteins to Address Cognitive Decline in AD
The study examined whether delivering mRNAs encoding synaptic proteins via extracellular vesicles (EVs) could enhance cognitive function in AD. Synaptic proteins such as SNAP25, GAP43, neurogranin, and synaptotagmin 1 are critical for memory and learning processes, and their depletion is a recognized characteristic of AD. A key obstacle addressed was the delivery of therapeutic agents across the blood-brain barrier, a capability naturally facilitated by EVs.
The research was conducted by Huimin Cai, Yana Pang, and colleagues from Xuanwu Hospital, Capital Medical University, and published in BMC Medicine in 2024.
Human and Mouse Studies on Synaptic Protein Dysregulation and Therapeutic Intervention
1. Experimental Process
- Recruitment of 57 Alzheimer’s disease (AD) patients and 56 healthy controls.
- Brain atrophy assessment using magnetic resonance imaging (MRI) and medial temporal lobe atrophy scoring.
- Measurement of synaptic protein levels (SNAP25, GAP43, neurogranin, and synaptotagmin 1) in plasma-derived extracellular vesicles (EVs) and cerebrospinal fluid (CSF).
- Analysis of the association between synaptic protein levels and hippocampal atrophy.
- Evaluation of synaptic protein levels in the brains of 5×FAD mice.
- Construction of rabies virus glycoprotein-engineered EVs loaded with mRNAs for GAP43 and SNAP25.
- Administration of engineered EVs to 5×FAD mice.
- Post-treatment assessments of synaptic protein levels, dendritic density, and cognitive function.
2. Key Experiments
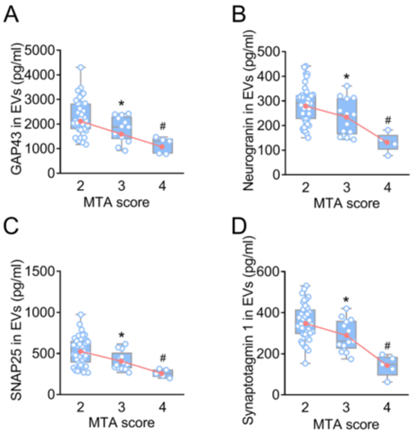
Experiment 1: Association of Synaptic Proteins with Brain Atrophy
- Procedure:
- A cohort of 57 AD patients and 56 healthy controls was recruited.
- Brain atrophy was assessed through MRI using the medial temporal lobe atrophy scoring system.
- Levels of synaptic proteins (SNAP25, GAP43, neurogranin, and synaptotagmin 1) were quantified in plasma-derived EVs and CSF.
- Participants were stratified by MTA scores to examine the relationship between synaptic protein levels and hippocampal atrophy.
- Result:
- Significant differences in synaptic protein levels were observed in both plasma-derived EVs and CSF across varying MTA scores in AD patients.
- A decrease in synaptic proteins was correlated with higher MTA scores.
- Finding:
- The study revealed that lower levels of synaptic proteins in EVs are associated with the severity of hippocampal atrophy in AD patients, suggesting their potential as biomarkers for the disease.
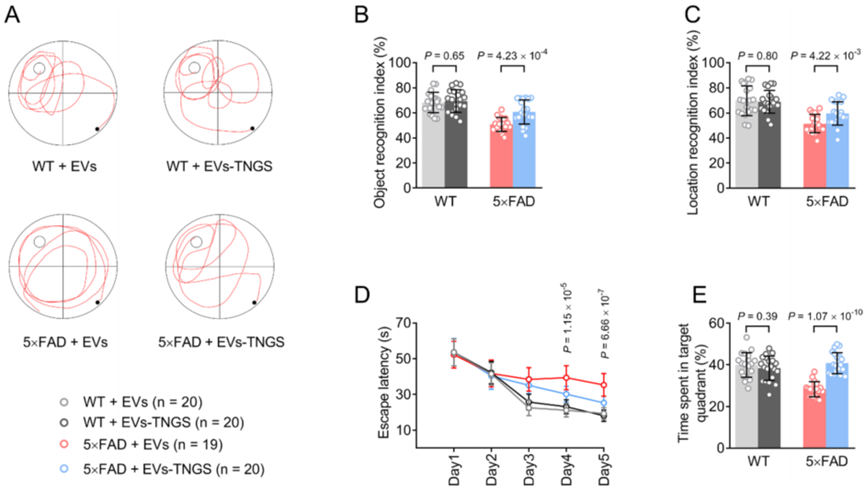
Experiment 2: Treatment with Engineered EVs
- Procedure:
- 5×FAD mice were used as a model for AD.
- Rabies virus glycoprotein-engineered EVs were constructed to deliver mRNAs for GAP43 and SNAP25.
- The engineered EVs were administered intravenously to 5×FAD mice at an optimal dosage.
- Post-treatment, the expression levels of GAP43 and SNAP25 were evaluated in hippocampal tissues, along with assessments of cognitive performance through behavioral tests.
- Result:
- The EV treatment led to a significant increase in both mRNA and protein levels of GAP43 and SNAP25 in the hippocampal tissues of treated mice.
- Cognitive performance improved in treated mice as evidenced by enhanced recognition index in the novel object recognition (NOR) and novel object location (NOL) tasks, as well as improved escape latencies in the Morris water maze (MWM).
- Finding:
- The administration of engineered EVs successfully upregulated synaptic protein levels and ameliorated cognitive impairment in the 5×FAD mouse model, highlighting the therapeutic potential of EV-based mRNA delivery in AD.
Implications for Alzheimer’s Disease Treatment
The research established that synaptic protein levels in EVs and CSF correspond to AD severity, as evidenced by brain atrophy in patients. In the 5xFAD mouse model, delivering GAP43 and SNAP25 mRNAs via engineered EVs raised synaptic protein expression, increased dendritic density, and improved cognitive function. This study marks the initial use of EV-mediated delivery of synaptic protein mRNAs in an AD mouse model, suggesting a potential approach for addressing synaptic dysfunction in AD.
Reference:
Cai, Huimin, et al. “Delivering synaptic protein mRNAs via extracellular vesicles ameliorates cognitive impairment in a mouse model of Alzheimer’s disease.” BMC medicine 22.1 (2024): 138.
