Editor:Sarah
A key obstacle in glioblastoma therapy is the BBTB, which blocks over 98% of chemotherapy drugs from entering the brain, rendering many potentially effective drugs unusable. OptoBBTB addresses this by using laser-activated, gold-based nanoparticles targeted to tight junction proteins in brain blood vessels. This method allows chemotherapeutic agents to cross the BBTB while minimizing damage to healthy tissues.
In a study published in Nature Communications, the team demonstrated that optoBBTB significantly increased the delivery of paclitaxel (Taxol)—a chemotherapeutic previously deemed ineffective in GBM due to poor brain penetration. Two genetically engineered mouse models were used to represent the diverse pathology of human glioblastoma: one exhibiting diffuse infiltrative growth (PS5A1), and another characterized by a solid, angiogenic tumor mass (73C).
The optoBBTB method boosted paclitaxel concentration in brain tumors by 16-fold and 5-fold in the infiltrative and angiogenic models, respectively. This increased drug access led to tumor volume reductions of up to 6-fold and extended median survival by 50% and 33% in the two models.
Unlike previously attempted strategies—such as osmotic agents or focused ultrasound—optoBBTB enables localized and reversible modulation of the BBTB with high spatial precision and without apparent long-term side effects.
Contributions and Key Findings
BBTB as a Barrier in GBM Treatment:
The study underscores that the BBTB is a significant factor limiting GBM treatment success.
Even regions of glioblastoma that appear to have disrupted vasculature still retain BBTB integrity in margins, limiting drug delivery.
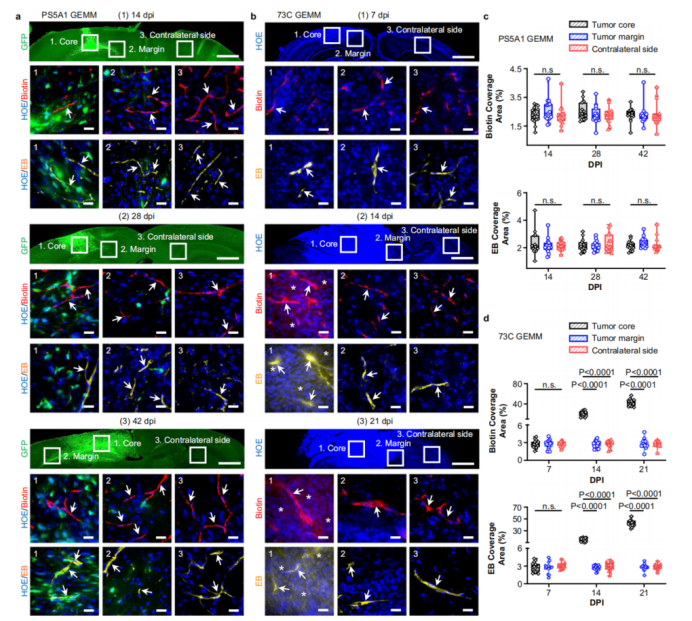
Figure 1: Blood-brain-tumor barrier heterogeneity in glioblastoma models.
Development of Two Distinct Mouse Models:
PS5A1 GEMM: Mimics diffusely infiltrative GBM, where tumor cells invade healthy brain tissue without inducing new blood vessel growth, maintaining intact BBTB.
73C GEMM: Models angiogenic GBM with irregular, leaky vasculature in the tumor core, reflecting heterogeneity seen in human cases.
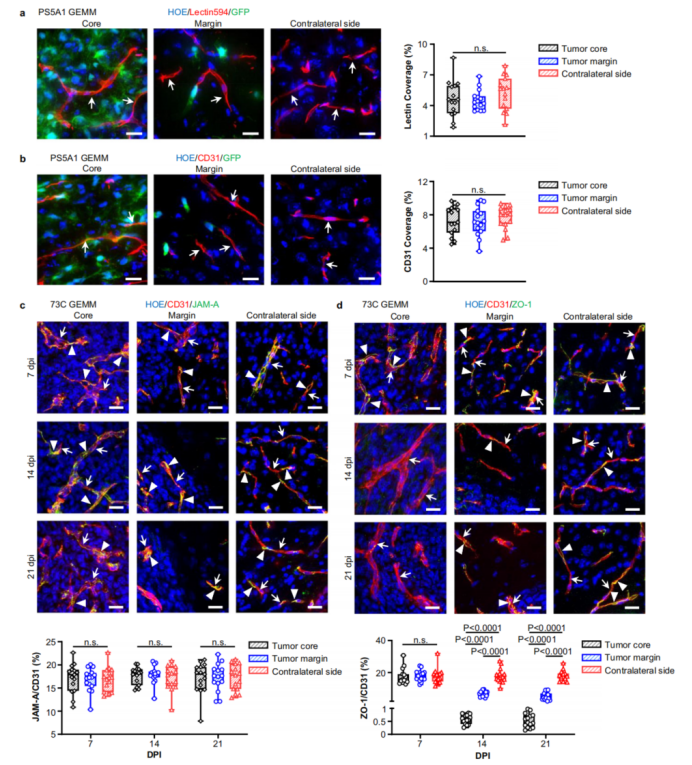
Figure 2: Tumor vascular structure and tight junction protein expression.
Mechanism of optoBBTB:
Uses 532nm picosecond laser pulses to stimulate gold nanoparticles bound to JAM-A (a tight junction protein), increasing permeability without heating or permanent damage.
Drug delivery enhancement is achieved through transient cytoskeletal remodeling, confirmed by lack of significant temperature increase and vessel damage post-treatment.
Efficacy of Paclitaxel with optoBBTB:
In PS5A1 mice: Paclitaxel concentration increased from 12 ng/g to 185 ng/g (16×), tumor volume decreased from 47 mm³ to 4 mm³, and median survival extended by 50%.
In 73C mice: Paclitaxel concentration increased from 240 ng/g to 1206 ng/g (5×), tumor volume decreased by over 2-fold, and median survival extended by 33%.
Treatment Safety:
No significant changes in body weight or evidence of neurotoxicity were observed during treatment cycles.
OptoBBTB was repeatable for multiple treatment sessions without inducing visible damage or inflammation in surrounding tissues.
Implications for Drug Repurposing:
The approach could re-enable the use of chemotherapies previously ruled out for GBM due to brain delivery limitations.
May support broader evaluation of potent anticancer agents for brain tumors when combined with delivery-enhancing methods.
Platform for Further Research:
The optical technique, with its spatial selectivity and repeatability, may serve as a preclinical platform for screening drugs in brain tumor therapy.
Future modifications—such as incorporating near-infrared light—could extend its applicability to deeper brain regions.
Reference
Cai, Qi, et al. “Optical Blood-Brain-Tumor Barrier Modulation Expands Therapeutic Options for Glioblastoma Treatment.” Nature Communications, vol. 14, no. 1, 2023, article no. 4934, https://doi.org/10.1038/s41467-023-40579-1.
