Editor: Tiffany
A leptin-conjugated Fas-blocking peptide, delivered systemically, inhibits apoptosis and reduces brain damage in animal models of ischemic stroke by targeting Fas-expressing regions, offering a potential new treatment strategy.
Key Highlights
- Research Question:
Can systemic delivery of a Fas-blocking peptide (FBP) inhibit Fas-mediated apoptosis in ischemic stroke, and does leptin conjugation enhance its delivery across the blood-brain barrier (BBB)? - Research Difficulties:
The BBB restricts drug access to the brain, limiting effective delivery of therapeutic agents for stroke, and larger PEG sizes can induce cellular toxicity, complicating peptide conjugation. - Key Findings:
Leptin-PEG-FBP reduces apoptosis by 40% in vitro, localizes to ischemic brain regions in mice, requires leptin receptor interaction for delivery, and decreases Fas expression by 50%, cleaved caspase-3 by 80%, and infarct volume by 70% in rat stroke models. - Innovative Aspects:
The study uses a 30-amino-acid leptin peptide to facilitate BBB penetration, conjugated with PEG to deliver FBP specifically to Fas-expressing ischemic brain regions, enabling systemic administration. - Importance of the Study:
This approach demonstrates a viable strategy for delivering therapeutics to the brain, potentially addressing the lack of effective treatments for ischemic stroke and other neurological disorders affected by BBB restrictions.
Ischemic Stroke and Current Treatment Challenges
Ischemic stroke occurs when blood flow to the brain is obstructed, leading to oxygen and nutrient deprivation in brain cells. This results in cell damage or death and subsequent neurological deficits. Symptoms include sudden numbness or weakness (often on one side of the body), confusion, difficulty speaking, vision problems, dizziness, and impaired coordination. These effects highlight the brain’s reliance on a consistent blood supply and the urgency of effective treatments.
The standard treatment for acute ischemic stroke is thrombolytic therapy using tissue plasminogen activator (tPA) to dissolve clots. However, tPA is effective only within a 3 to 4.5-hour window from stroke onset, and its use is limited to 3.4-5.2% of eligible patients due to timing constraints or medical contraindications. Beyond tPA, options are primarily supportive, as the blood-brain barrier (BBB) poses a significant obstacle to delivering therapeutic agents to the brain. This limitation has driven research into alternative treatment strategies.
Targeting Fas-Mediated Apoptosis with Leptin-Conjugated Peptide
A team of researchers, including Sungeun Chung, Yujong Yi, Irfan Ullah, and collaborators from Hanyang University and other institutions, sought to develop a new approach to treat ischemic stroke. Their goal was to inhibit Fas-mediated apoptosis—a key contributor to neuronal death in stroke—by delivering a Fas-blocking peptide (FBP) to the brain. To bypass the BBB, they conjugated FBP with a leptin-derived peptide known to aid brain delivery. This study, published in the International Journal of Molecular Sciences in January 2024, tested the approach in animal models.
Experimental Design and Key Outcomes
The study employed in vitro and in vivo experiments to evaluate the efficacy and delivery of the leptin-PEG-FBP peptide.
1. In Vitro Experiment: Inhibition of Apoptosis in Neuronal Cells
- Procedure: Mouse neuroblastoma cells were subjected to hypoxic conditions to mimic stroke-related oxygen deprivation. The cells were treated with leptin-PEG-FBP and exposed to Fas ligand (FasL), which triggers apoptosis through the Fas receptor.
- Result: Treatment reduced Annexin V-positive cells by 40% compared to controls, indicating a decrease in apoptosis.
- Finding: The leptin-PEG-FBP peptide preserves FBP’s ability to inhibit Fas-mediated apoptosis in hypoxic neuronal cells.
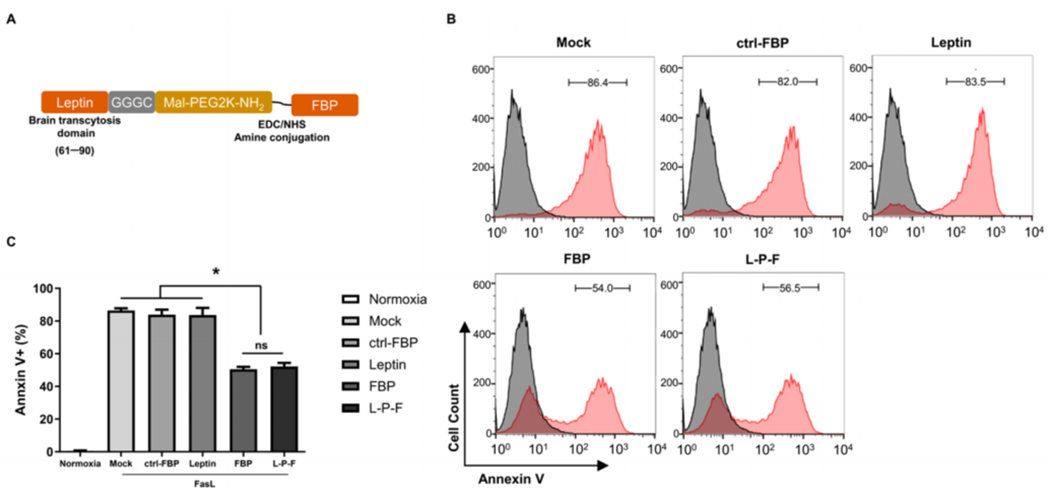
Figure 1. The inhibitory effect of FBP or leptin-PEG-FBP (L-P-F) on FasL-induced apoptosis in hypoxic-N2a.
2. In Vivo Experiment: Localization in Mouse Stroke Models
- Procedure: Photothrombosis was used to induce localized ischemic stroke in mice. The leptin-PEG-FBP peptide, tagged with a fluorescent marker, was administered intravenously.
- Result: Fluorescence imaging revealed peptide accumulation in the ischemic brain region, correlating with elevated Fas expression.
- Finding: The peptide crosses the BBB and localizes to the ischemic area, leveraging leptin-mediated transport.
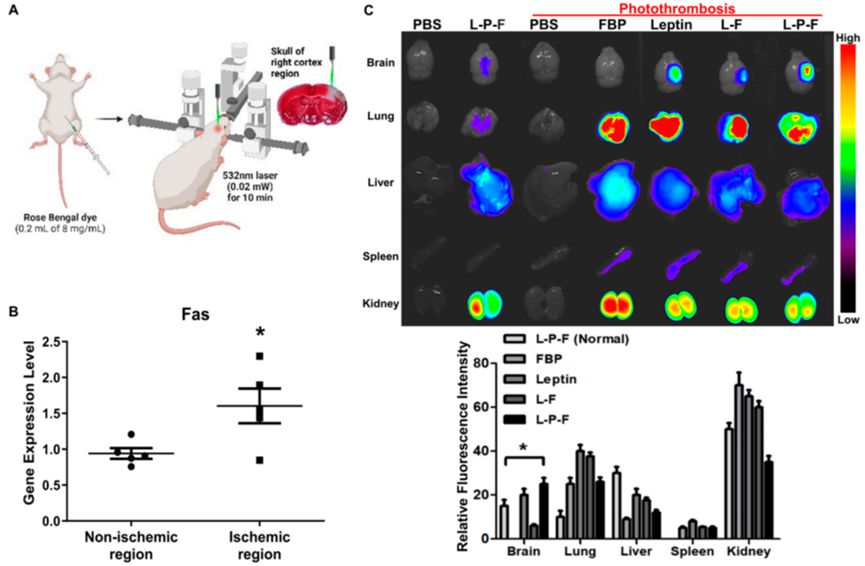
Figure 2. Systemically delivered leptin-PEG-FBP (L-P-F) localizes to Fas-expressing brain regions in the photothrombotic mouse model of ischemia.
3. Validation with Leptin Receptor-Deficient Mice
- Procedure: The same experiment was conducted in leptin receptor-deficient (db/db) mice following photothrombotic stroke, with the peptide administered intravenously.
- Result: No fluorescence was observed in the brains of db/db mice, indicating the peptide did not reach the target region.
- Finding: Delivery to the brain depends on the leptin-leptin receptor interaction, confirming the mechanism’s specificity.
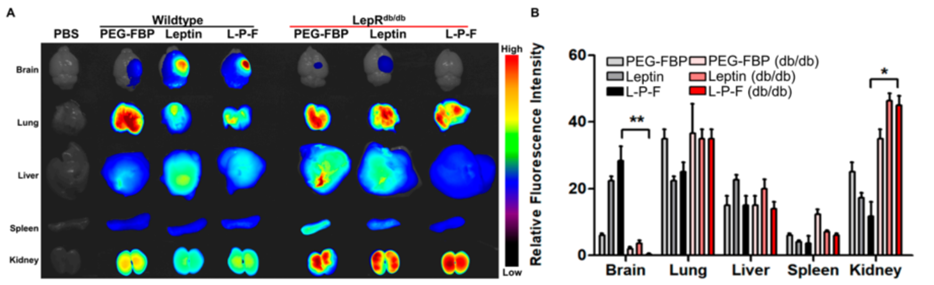
Figure 3. Leptin–leptin receptor binding facilitates Leptin-PEG-FBP delivery to the brain.
4. Therapeutic Effects in Rat Stroke Models
- Procedure: Middle cerebral artery occlusion (MCAO) was performed in rats to simulate human stroke. Rats received leptin-PEG-FBP, and brain tissue was assessed 48 hours post-treatment.
- Result: Treated rats showed a 50% reduction in Fas expression, an 80% decrease in cleaved caspase-3 (an apoptosis indicator), and a 70% reduction in infarct volume compared to controls. Tissue morphology also improved.
- Finding: The peptide reaches the brain, reduces apoptosis, and limits infarct size in a relevant stroke model.
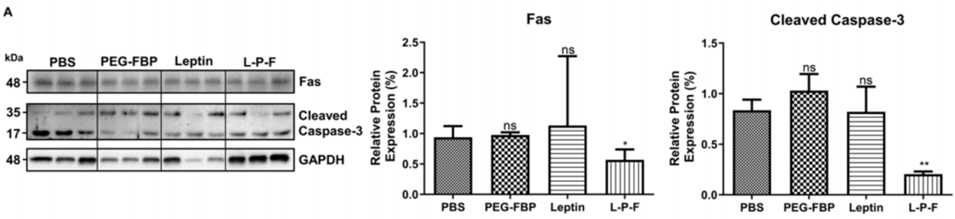
Figure 4. Representative Western blots and quantification of protein expression for Fas and cleaved caspase-3 in the brain of MCAO mice at 48 h post-MCAO.
Implications for Ischemic Stroke Therapy
The study demonstrates that leptin-PEG-FBP, delivered systemically, inhibits Fas-mediated apoptosis in ischemic brain regions, reducing neuronal death and infarct volume in animal models of stroke. The leptin conjugation enables the peptide to cross the BBB and target affected areas. These findings indicate a potential new strategy for ischemic stroke treatment, possibly extending the therapeutic window beyond current options. Further studies are required to assess its applicability and safety in humans.
Reference:
Chung, Sungeun, et al. “Systemic treatment with Fas-blocking peptide attenuates apoptosis in brain ischemia.” International Journal of Molecular Sciences 25.1 (2024): 661.
