Editor: Tiffany
Researchers have discovered that combining temozolomide and doxorubicin significantly enhances the effectiveness of glioblastoma treatment, particularly in drug-resistant cells, offering new hope for patients battling this challenging cancer.
Key Highlights
- Research Question:
Can the combination of temozolomide and doxorubicin improve treatment outcomes for glioblastoma multiforme, especially in cases resistant to temozolomide? - Research Difficulties:
Glioblastoma multiforme is notoriously difficult to treat due to its aggressive nature and the development of resistance to conventional therapies. - Key Findings:
The combination therapy not only inhibited the growth of glioblastoma cells but also increased doxorubicin uptake and apoptosis in resistant cell lines. - Innovative Aspects:
This study demonstrates a synergistic effect between two established drugs, suggesting a novel approach to enhance treatment efficacy without increasing toxicity. - Importance of the Study:
The findings may lead to improved therapeutic strategies for glioblastoma patients, potentially increasing survival rates and quality of life.
Understanding Glioblastoma and Current Treatment Limitations
Glioblastoma Multiforme (GBM) is a formidable adversary in the world of oncology. Classified as a grade IV brain tumor by the World Health Organization, GBM is the most aggressive type of brain cancer and accounts for 60% of all brain tumors in adults. This malignancy predominantly strikes older individuals, with a median diagnosis age of 64, and affects men more frequently than women. Its symptoms—headaches, seizures, and cognitive decline—often emerge late, when the tumor has already advanced significantly, complicating early detection and intervention.
The standard treatment for GBM involves a multi-pronged approach: surgical resection to remove as much of the tumor as possible, followed by radiation and chemotherapy with Temozolomide, a drug capable of crossing the blood-brain barrier to target cancer cells. Despite this aggressive regimen, the prognosis remains grim, with a median survival of just 14 to 16 months. The tumor’s invasive growth into surrounding brain tissue and its tendency to develop resistance to Temozolomide are key reasons why current therapies fall short, leaving patients and clinicians desperate for more effective solutions.
Objective: Evaluating the Synergistic Potential of Temozolomide and Doxorubicin
A study recently tackled one of the biggest hurdles in GBM treatment: resistance to Temozolomide. The research, led by Laxmi Dhungel, Mandy E. Rowsey, Cayla Harris, and Drazen Raucher from the Department of Cell and Molecular Biology at the University of Mississippi Medical Center, aimed to explore whether pairing Temozolomide with Doxorubicin—a widely used chemotherapeutic drug—could overcome this resistance and boost treatment efficacy. Their goal was to determine if this drug combination could work synergistically, offering hope even to patients with Temozolomide-resistant tumors. The findings, published in the journal Molecules in 2024, shed light on a promising new strategy for combating this deadly disease.
Experimental Design and Key Findings
The study explored the effects of Temozolomide, Doxorubicin, and their combination on Glioblastoma Multiforme (GBM) cells using three cell lines: U87 (Temozolomide-sensitive), GBM43, and GBM6 (Temozolomide-resistant). The experimental procedures included cell viability assays with an MTT assay, synergy determination using SynergyFinder, Doxorubicin uptake measurement via flow cytometry, apoptosis assays with annexin V/PI staining, and cell cycle analysis with propidium iodide staining. These methods assessed how the drugs, alone and combined, impacted cell survival, drug interactions, uptake, cell death, and proliferation in GBM cells.
Cell Viability Assays
- Procedure: U87, GBM43, and GBM6 cells were treated with Temozolomide (40-320 μM), Doxorubicin (5-80 nM), or both for five days. Cell survival was measured using an MTT assay, which quantifies metabolic activity.
- Result: The combination treatment significantly reduced cell survival compared to single treatments across all cell lines, including the resistant GBM43 and GBM6 lines.
- Finding: Combining Temozolomide and Doxorubicin enhances the inhibitory effect on GBM cells, even in those resistant to Temozolomide, suggesting a promising approach to overcome resistance.
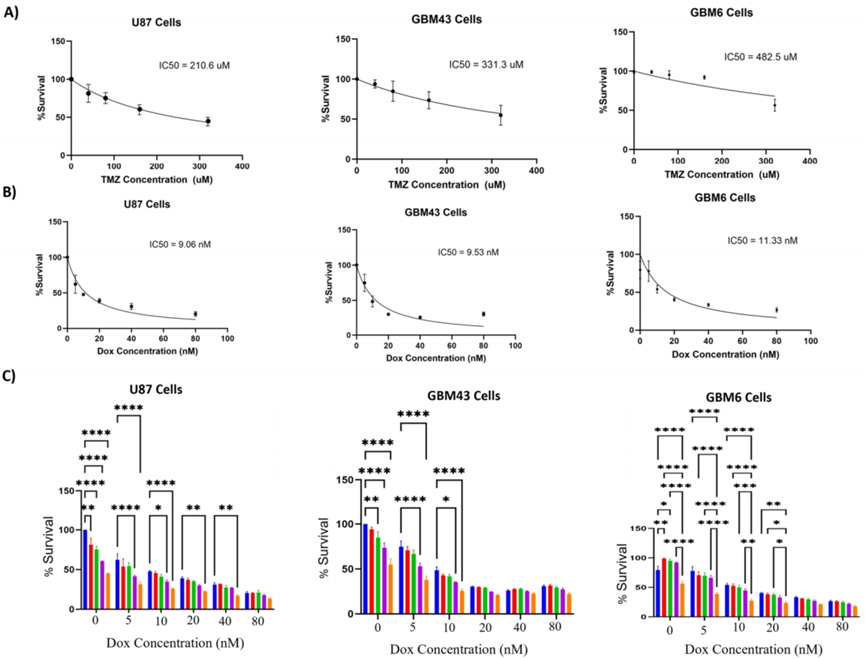
Figure 1. Effect of combination treatment with temozolomide and doxorubicin.
Synergy Determination
- Procedure: Drug interactions were analyzed using SynergyFinder, evaluating combinations of Temozolomide and Doxorubicin with models like HSA, Bliss, Loewe, and ZIP to determine synergy.
- Result: Synergy was evident at specific concentrations, such as Temozolomide (160 μM) with Doxorubicin (5-10 nM), according to the HSA model, with no antagonism observed.
- Finding: The combination demonstrates synergistic effects, indicating improved efficacy without adverse interactions, which could benefit GBM treatment.
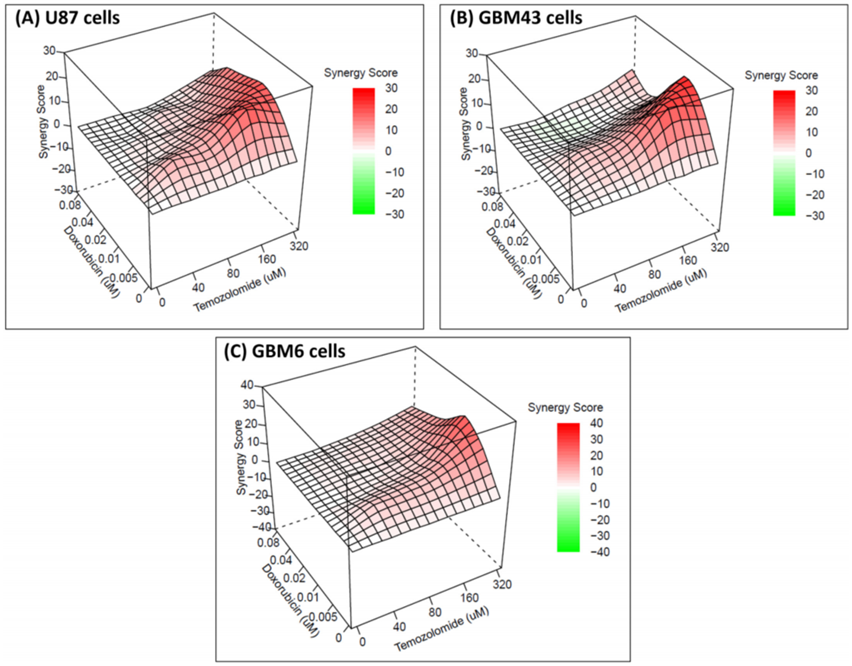
Figure 2. The 3D images showing synergy between the combination of drugs (temozolomide and doxorubicin) based on HSA score for different cell lines.
Doxorubicin Uptake
- Procedure: GBM43 cells were treated with Doxorubicin (10 nM) alone or with Temozolomide (160 μM) for 24 hours. Flow cytometry measured Doxorubicin uptake based on its fluorescence.
- Result: The combination increased Doxorubicin uptake by 1.6-fold compared to Doxorubicin alone in GBM43 cells.
- Finding: Temozolomide boosts Doxorubicin uptake in resistant cells, likely enhancing the combination’s ability to kill cancer cells.
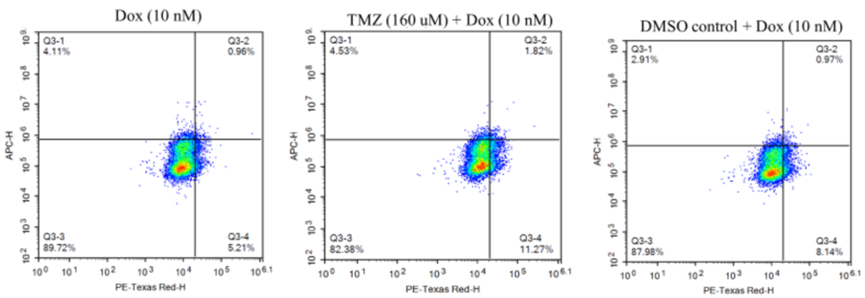
Figure 3. Representative image showing flow cytometry results for doxorubicin uptake. Cells that were detected on PE Texas red channel but not on APC channel (Q3-4) were considered positive for doxorubicin uptake.
Apoptosis Assays
- Procedure: GBM43 cells were treated with Temozolomide (160 μM), Doxorubicin (10 nM), or both for 24 hours. Apoptosis was assessed using annexin V/PI staining and flow cytometry.
- Result: The combination induced four times more total apoptosis than Temozolomide alone and 1.5 times more than Doxorubicin alone.
- Finding: The combination significantly increases programmed cell death in resistant GBM cells, reinforcing its potential to combat resistance.
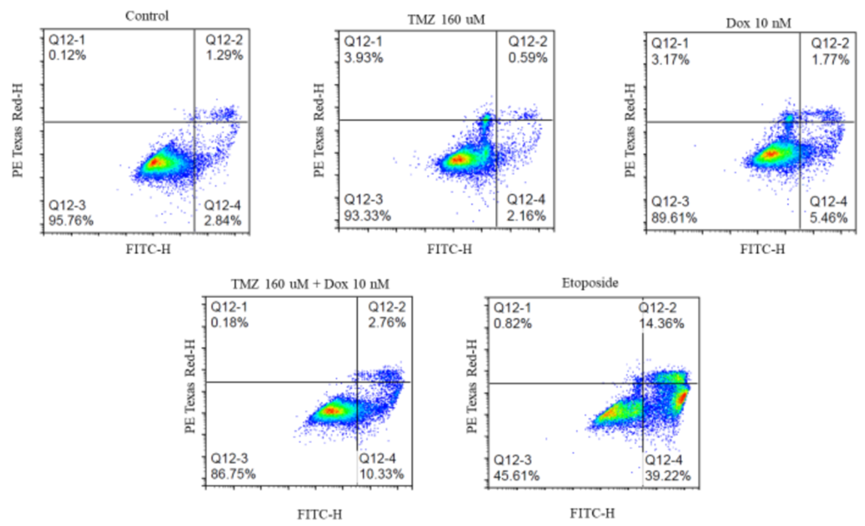
Figure 4. Effect of different treatments on apoptosis of GBM43 cells.
Cell Cycle Analysis
- Procedure: GBM43 cells were treated with Temozolomide (160 μM), Doxorubicin (10 nM), or both for 48 hours. Cell cycle phases were analyzed using propidium iodide staining and flow cytometry.
- Result: Doxorubicin and the combination caused higher G2 phase arrest, while Temozolomide alone showed reduced G2 arrest in resistant cells.
- Finding: The combination retains Doxorubicin’s cell cycle arrest effect at the G2 phase, critical for halting cancer cell proliferation in resistant lines.
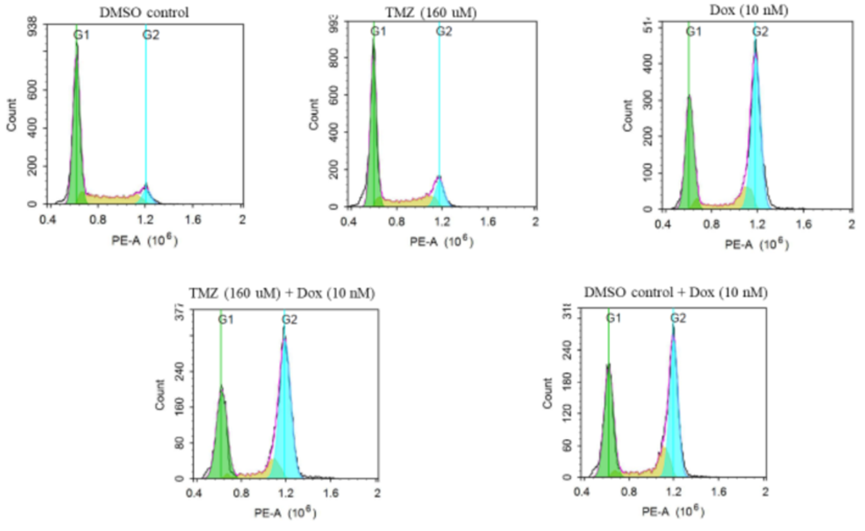
Figure 5. Representative image showing cell cycle arrest for different treatment groups. The cells were stained with PI and detected on PE channel on flowcytometry.
Conclusions on Combination Therapy Efficacy and Future Directions
This study unveils a promising advance in GBM treatment: combining Temozolomide with Doxorubicin yields synergistic effects that could transform outcomes, even for patients with resistant tumors. The research shows that this pairing slashes cell viability, boosts Doxorubicin uptake, ramps up apoptosis, and maintains cell cycle arrest—collectively delivering a powerful blow to GBM cells. What’s innovative here is the use of two existing drugs in a novel combination to sidestep Temozolomide resistance, a major barrier in current therapy. This approach could pave the way for more effective treatments and longer survival for GBM patients. Moreover, the study hints at practical solutions to Doxorubicin’s limitation—it doesn’t naturally cross the blood-brain barrier—by suggesting local delivery methods like hydrogel systems. This could make the combination viable in clinical settings.
Reference:
Dhungel, Laxmi, et al. “Synergistic effects of temozolomide and doxorubicin in the treatment of glioblastoma multiforme: enhancing efficacy through combination therapy.” Molecules 29.4 (2024): 840.
