Editor: Tiffany
Introduction
Infected wounds, particularly in the oral and maxillofacial regions, pose a significant challenge in clinical settings. These wounds often arise from trauma that exposes skin and oral tissues to bacteria, leading to infections that delay healing and result in scarring. Conventional treatments, while somewhat effective, encounter issues such as antibiotic resistance and excessive inflammation. A 2024 study from the International Journal of Oral Science presents a hybrid hydrogel made of tetrahedral framework nucleic acids (tFNAs) and hyaluronic acid-methacrylic anhydride (HAMA), combined with the antimicrobial peptide GL13K. This article reviews the study’s background, methods, and results, focusing on its contributions to wound care.
Background
The Problem of Infected Wounds
Infected wounds are common, especially in areas like the mouth and face where soft tissue injuries occur frequently. Bacteria can invade these wounds, causing infections that slow healing and lead to complications. Symptoms include redness, swelling, pain, pus, and, in severe cases, fever. If not adequately addressed, these infections can cause noticeable scarring, impacting both function and appearance.
Limitations of Current Treatments
Standard approaches to infected wounds involve mechanical debridement (removal of dead tissue), suturing, antibiotics, and scar reduction techniques like laser therapy. However, these methods face several challenges:
- Antibiotic Resistance: Increased bacterial resistance due to frequent antibiotic use reduces treatment effectiveness.
- Inflammation: Excessive inflammation can worsen tissue damage and prolong healing.
- Scarring: Even with advanced methods, scarring remains a common outcome.
These issues highlight the need for an alternative approach that simultaneously manages infection, inflammation, and healing.
Research Overview
Aims and Challenges
The research team, led by Cai Qi and Yunfeng Lin from Sichuan University’s State Key Laboratory of Oral Diseases, sought to develop a hydrogel that:
- Eliminates bacteria without traditional antibiotics
- Reduces inflammation
- Supports faster healing with reduced scarring
The study addressed key difficulties, including bacterial resistance and suboptimal healing outcomes, which are persistent problems in conventional treatments.
The Approach: HAMA/t-GL13K Hydrogel
The researchers developed a hybrid hydrogel combining tFNAs—DNA nanostructures—with HAMA, a biocompatible material, and GL13K, an antimicrobial peptide. This formulation was designed as an injectable treatment adaptable to different wound shapes, aiming to overcome limitations of existing therapies.
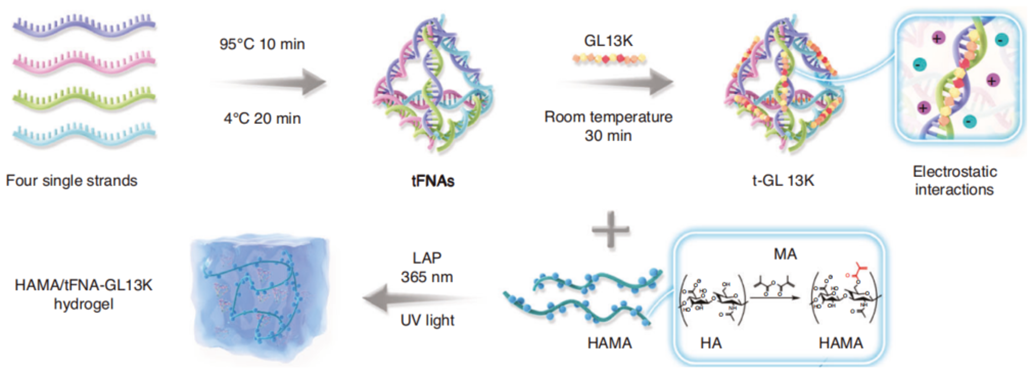
Figure 1. Schematic of the HAMA/t-GL13K hybrid hydrogel, illustrating the structural integration of tFNAs, HAMA, and GL13K.
Methodology
Creating the Hydrogel
The hydrogel was prepared through a multi-step process:
- tFNAs Synthesis: DNA strands were self-assembled into tetrahedral structures and combined with GL13K to form t-GL13K.
- Hydrogel Formation: HAMA was photocross-linked with t-GL13K to create the final hydrogel.
- Testing: The hydrogel’s antibacterial, anti-inflammatory, and healing properties were evaluated in laboratory and animal studies.
Experiments
The study conducted three main experiments:
1. Antibacterial Efficacy
- Method: Tested against E. coli and S. aureus using agar diffusion and colony counting.
- Outcome: Demonstrated a substantial decrease in bacterial growth, indicating effective broad-spectrum antibacterial activity.
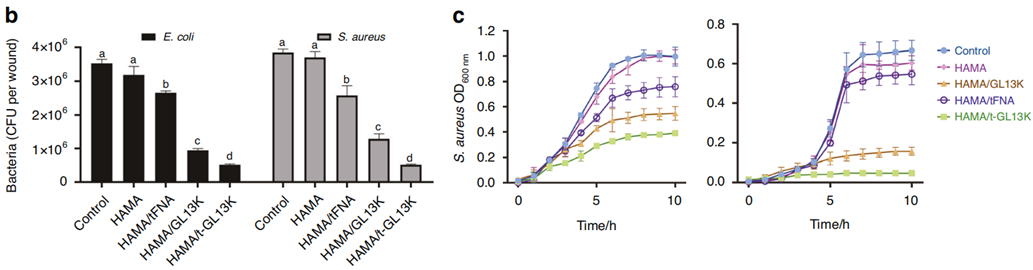
Figure 2. (b) Count bacteria of CFU of E. coli and S. aureus. © Growth curves of E. coli and S. aureus in different hydrogels treatment.
2. Anti-Inflammatory Effects
- Method: Assessed reactive oxygen species (ROS) and inflammatory markers in cell cultures.
- Outcome: Showed reduced ROS and cytokine levels, suggesting a decrease in inflammation.
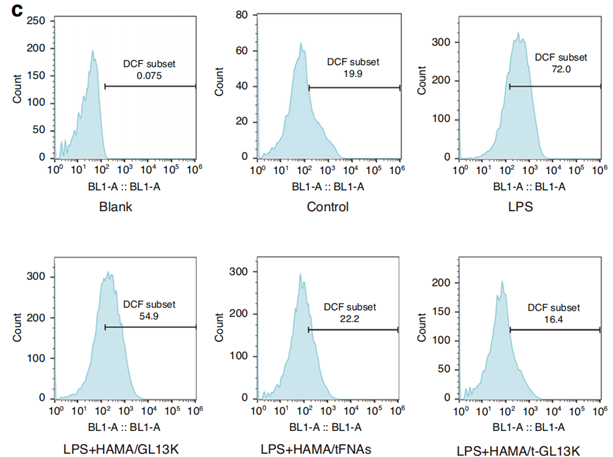
Figure 3. Quantification of ROS in HaCaT cells.
3. Wound Healing
- Method: Applied to infected, full-thickness skin defects in rats.
- Outcome: Observed faster wound closure and less scarring compared to untreated wounds.
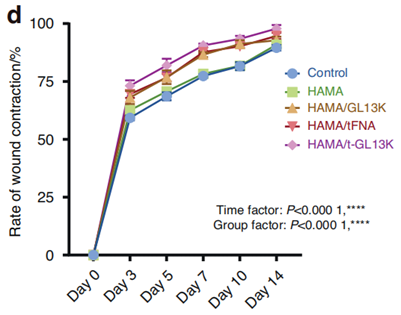
Figure 4. Wound healing rate of diverse groups.
Results and Findings
Antibacterial Activity
The hydrogel effectively reduced bacterial presence in tested samples, providing an alternative to antibiotics that avoids resistance issues. This is notable given the global rise in antibiotic-resistant bacteria.
Inflammation Control
By lowering ROS and inflammatory signals, the hydrogel reduced tissue damage, which likely contributed to improved healing rates.
Healing Outcomes
In animal models, wounds treated with the hydrogel closed more quickly and exhibited reduced scarring, indicating potential benefits for both functional and aesthetic recovery.
Discussion
Significance
The HAMA/t-GL13K hydrogel targets key aspects of infected wound management:
- Infection Control: GL13K offers an antibacterial effect independent of traditional antibiotics.
- Inflammation Reduction: tFNAs and HAMA decrease inflammation levels.
- Healing Support: The combination enhances tissue regeneration with less scarring.
Its injectable form and rapid curing properties make it suitable for clinical application, particularly for irregularly shaped wounds.
Future Applications
The hydrogel’s structure suggests possible uses beyond wound healing, such as in tissue engineering for scaffolds or organoids. As antibiotic resistance increases, nucleic acid-based treatments like this may gain importance in medical practice.
Conclusion
The HAMA/t-GL13K hybrid hydrogel, developed by researchers at Sichuan University, offers a comprehensive approach to managing infected wounds. By addressing antibiotic resistance, reducing inflammation, and improving healing, it provides a practical option for a challenging clinical issue. This study contributes valuable insights to wound care and lays the groundwork for further research into its broader applications, potentially enhancing patient outcomes in the future.
Reference:
Qi, Cai, et al. “Tetrahedral framework nucleic acids/hyaluronic acid-methacrylic anhydride hybrid hydrogel with antimicrobial and anti-inflammatory properties for infected wound healing.” International Journal of Oral Science 16.1 (2024): 30.
