Editor: Sarah
Summary: A collaborative study from Tokushima University, Nagoya University, and Zagazig University introduces a promising advancement in melanoma therapy by combining iontophoresis technology with a minimal mRNA vaccine. This approach addresses the longstanding challenge of efficient mRNA delivery and offers potential for improved cancer treatment strategies.
Can Transdermal mRNA Vaccine Delivery Revolutionize Melanoma Treatment?
mRNA vaccines have garnered significant attention for their potential in cancer immunotherapy, particularly in the wake of their success in treating infectious diseases. However, the delivery of these vaccines remains a significant challenge, particularly for complex treatments like melanoma. A recent study involving researchers from Tokushima University, Nagoya University, and Zagazig University explored the combination of iontophoresis (IP) technology and a minimal mRNA vaccine as a potential solution to this problem.
The Challenge of mRNA Vaccine Delivery in Cancer Therapy
mRNA vaccines are widely regarded for their ability to target specific tumor antigens, prompting immune responses that can combat cancer. Despite this potential, mRNA vaccines face challenges due to their large size, instability, and negative charge, which make it difficult for them to penetrate the skin and reach immune cells effectively. These issues have hindered the widespread use of mRNA vaccines in cancer therapies, including melanoma.
A New Approach: Iontophoresis and mRNA Vaccines
In this study, the researchers combined iontophoresis, a technique that uses a low electrical current to help molecules pass through the skin, with a minimal mRNA vaccine encoding a melanoma-specific tumor antigen. The results were promising, demonstrating the effective transdermal delivery of the mRNA vaccine and the stimulation of a robust immune response in melanoma-bearing mice.
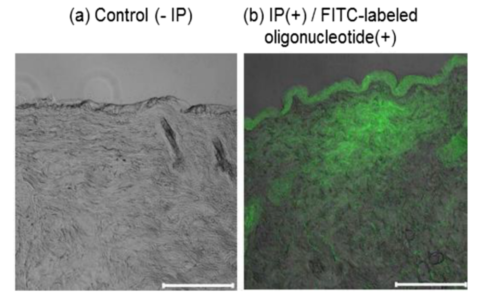
Figure 1: Intradermal Distribution of FITC-Labeled Oligonucleotide.
Key Findings:
Efficient mRNA Delivery: Iontophoresis enabled the minimal mRNA vaccine to penetrate the skin and effectively reach immune cells. The study showed that the IP-enhanced delivery system was successful in delivering the vaccine to skin-resident immune cells, significantly enhancing its therapeutic potential.
Tumor Regression in Mice: Mice treated with the mRNA vaccine via iontophoresis experienced notable tumor inhibition. This result provided direct evidence of the therapeutic potential of the approach for melanoma treatment.
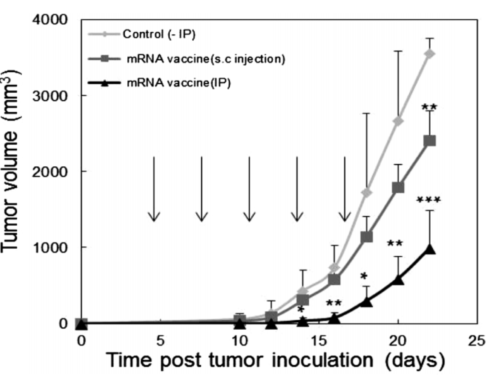
Figure 2: Effect of Immunization with mRNA Vaccines on Tumor Inhibition.
Enhanced Immune Activation: The combination of iontophoresis and the mRNA vaccine led to the activation of key immune cells, particularly cytotoxic CD8+ T cells, which play a vital role in targeting and eliminating tumor cells. The treatment also increased the expression of key cytokines such as interferon-gamma (IFN-γ), further bolstering the immune response.
Methodology
The researchers utilized iontophoresis to deliver a minimal mRNA vaccine encoding the melanoma-associated gp100(25–33) peptide to a mouse model. This non-invasive method allowed for the delivery of the vaccine through the outer layers of the skin, avoiding the discomfort and complications associated with traditional injection-based delivery methods.
Contributions and Key Findings:
Efficient Transdermal Delivery: Iontophoresis technology enabled the mRNA vaccine to penetrate the skin barrier. By using a weak electrical current (0.17 mA/0.5 cm² for 1 hour), the researchers were able to deliver the mRNA to skin-resident immune cells, including Langerhans cells and dendritic cells. This approach circumvented some of the inherent challenges of direct vaccine injection and allowed for a more targeted immune response.
Activation of Immune Cells: The transdermal delivery method led to the activation of cytotoxic CD8+ T cells in the treated mice, which are crucial for attacking and eliminating cancerous cells. Additionally, other immune cells, including CD4+ T cells, also infiltrated the tumor tissue, contributing to the tumor suppression observed.
Cytokine Expression: The combination of iontophoresis and mRNA delivery resulted in a marked increase in the expression of important cytokines such as IFN-γ and tumor necrosis factor (TNF)-α. These cytokines play significant roles in regulating immune responses and promoting the immune system’s ability to target cancer cells. This increase in cytokine production was particularly evident in tumor tissues, highlighting the effectiveness of the approach in stimulating an anti-cancer immune response.
Tumor Regression and Immune System Activation: The IP-assisted mRNA delivery not only resulted in increased immune cell activation but also led to a significant reduction in tumor volume in the mouse model. The results demonstrated that the combined approach of iontophoresis and minimal mRNA vaccines could be a viable strategy for melanoma treatment.
Comparison with Other Delivery Methods: Traditional subcutaneous injection of the mRNA vaccine did not show significant effects on tumor volume except at later stages of treatment. In contrast, the iontophoresis method showed more consistent and early-stage tumor regression, demonstrating its superior potential for enhanced mRNA delivery and immune activation.
Implications for Cancer Treatment
This study presents a promising new strategy for melanoma immunotherapy. The ability to deliver mRNA vaccines via iontophoresis could make cancer treatments less invasive and more accessible. Furthermore, the approach holds the potential to be applied to other types of cancer, providing a novel avenue for immunotherapy that minimizes the need for invasive procedures and injections.
Conclusion
The research provides strong evidence for the potential of iontophoresis-enhanced transdermal delivery of mRNA vaccines in treating melanoma. By improving the delivery of mRNA vaccines to immune cells within the skin, this method enhances the immune response and offers a less invasive alternative to traditional delivery systems. As research continues, this approach could become an integral part of future cancer treatment strategies, particularly for skin cancers like melanoma.
Reference
Husseini, Rabab A., et al. “Use of Iontophoresis Technology for Transdermal Delivery of a Minimal mRNA Vaccine as a Potential Melanoma Therapeutic.” Biological and Pharmaceutical Bulletin, vol. 46, no. 2, 2023, pp. 301-308. https://doi.org/10.1248/bpb.b22-01097.
