Editor: Nina
Scientists develop pH-gated nanoparticles that selectively reprogram tumor-associated macrophages to enhance anti-tumor immune responses, offering a novel strategy for targeted cancer immunotherapy.
Key Preview
- Research Question: The study investigates how to selectively reprogram tumor-associated macrophages (TAMs), particularly M2-like TAMs, to enhance their anti-tumor immune responses while minimizing impacts on healthy tissue.
- Research Design and Strategy: Researchers designed pH-gated nanoparticles (PGNs) that specifically target the acidic lysosomal environment of M2-like TAMs in tumors. This selective targeting aims to switch these macrophages to an M1-like phenotype, thereby improving their ability to cross-present antigens.
- Method: The study employed a library of PGNs with varying pH sensitivities, identifying PGN4.9 as the most effective at targeting M2-like TAMs. In vitro and in vivo experiments evaluated the ability of these nanoparticles to modulate macrophage phenotypes and enhance immune responses.
- Key Results: PGN4.9 successfully converted M2-like TAMs to M1-like macrophages, decreasing lysosomal acidity and enhancing antigen cross-presentation. This resulted in sustained tumor regression in mouse models.
- Significance of the Research: The findings provide valuable insights into targeted cancer immunotherapy strategies by selectively regulating macrophage function in the tumor microenvironment, potentially improving patient outcomes in cancer treatment.
Introduction
Cancer remains one of the leading causes of morbidity and mortality worldwide, characterized by uncontrolled cell growth that can invade surrounding tissues and metastasize to distant sites. The complexity and heterogeneity of tumors pose significant challenges for effective treatment, necessitating the exploration of innovative therapeutic strategies. Traditional cancer therapies, including chemotherapy and radiation, often employ systemic drug delivery methods that aim to target tumor cells. However, these approaches frequently result in off-target effects, leading to damage in healthy tissues and severe side effects, which can compromise patient quality of life and treatment efficacy.
One common strategy in conventional cancer treatment involves the use of chemotherapeutic agents that circulate throughout the body. These drugs are designed to preferentially target rapidly dividing cancer cells; however, their lack of specificity often leads to significant toxicity in normal tissues, particularly in the bone marrow, gastrointestinal tract, and hair follicles. This systemic exposure can result in a range of adverse effects, including nausea, immunosuppression, and hair loss, which contribute to treatment discontinuation and reduced overall survival rates.
Moreover, the tumor microenvironment often harbors immunosuppressive cells, such as tumor-associated macrophages (TAMs), which can adopt a pro-tumorigenic M2-like phenotype. These M2-like TAMs not only facilitate tumor progression but also inhibit effective anti-tumor immune responses, further complicating treatment outcomes. Current therapeutic strategies struggle to effectively reprogram these macrophages without adversely affecting healthy immune cells, leading to a persistent challenge in achieving durable clinical responses in cancer patients.
To address these limitations, researchers are exploring innovative drug delivery strategies that enhance the selectivity and efficacy of cancer therapies. One such approach is the use of pH-gated nanoparticles (PGNs), which can selectively target the acidic lysosomal environment characteristic of M2-like TAMs within tumors. By harnessing the unique properties of these nanoparticles, it is possible to reprogram M2-like macrophages into M1-like phenotypes, thereby promoting a more effective anti-tumor immune response while minimizing systemic toxicity. This novel strategy represents a significant advancement in cancer immunotherapy, offering a promising pathway to improve patient outcomes through targeted modulation of the tumor microenvironment.
Research Team and Aim
The research was conducted by a team from Peking University, led by Mingmei Tang, with significant contributions from co-authors Binlong Chen and Yiguang Wang, among others. The study was carried out in 2023, focusing on novel approaches to cancer immunotherapy. The findings were published in the paper titled “pH-gated nanoparticles selectively regulate lysosomal function of tumour-associated macrophages for cancer immunotherapy,” which appeared in Nature Communications.
The aim of the research, as explained by lead researcher Mingmei Tang, was to “develop a targeted nanoadjuvant that could specifically modulate the lysosomal function of M2-like TAMs, facilitating their reprogramming into M1-like macrophages to improve their ability to present antigens and elicit anti-tumor immune responses.” This objective highlights the team’s focus on addressing the challenges posed by the tumor microenvironment in cancer treatment.
Experimental Process
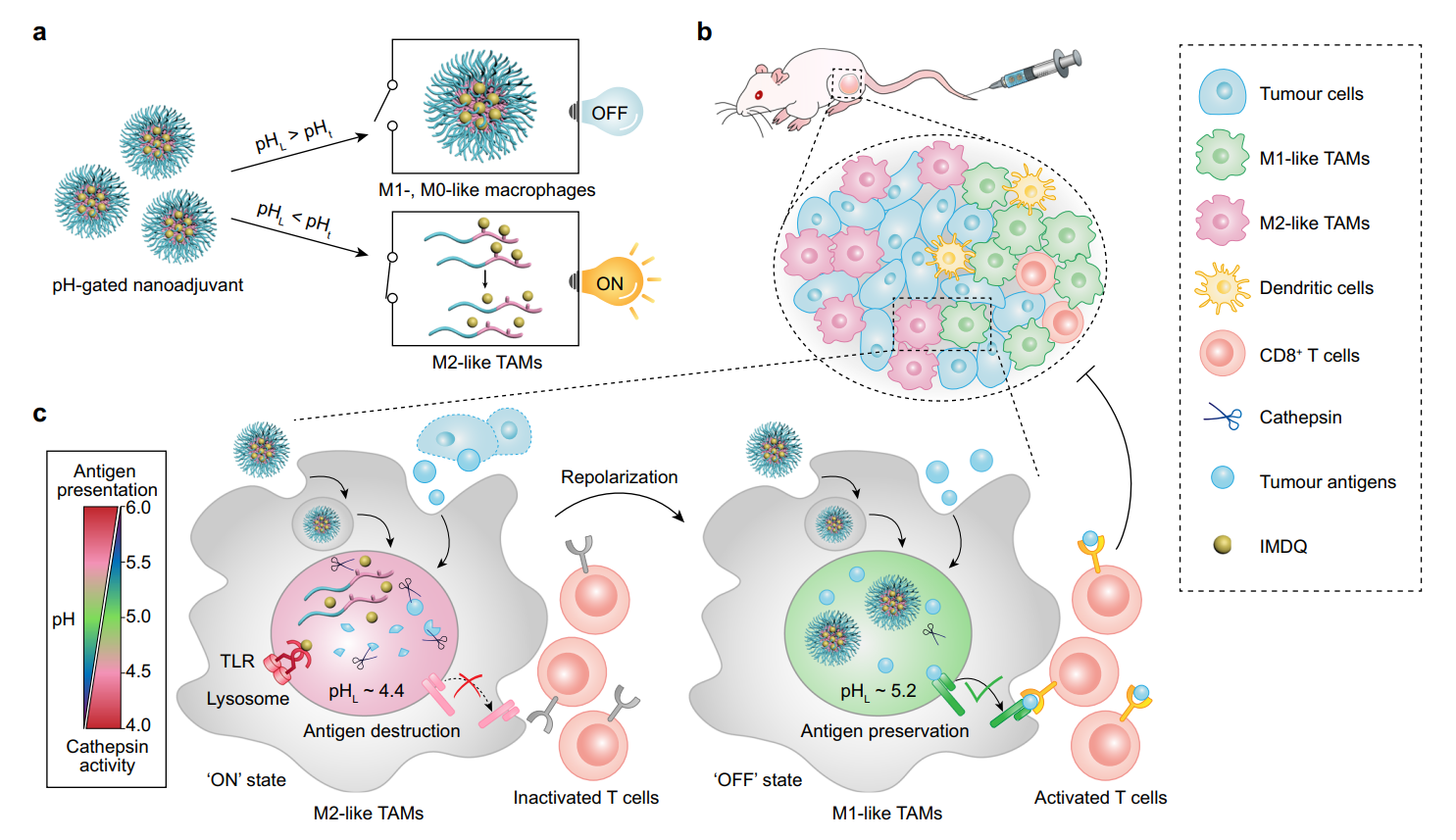
Figure 1. Design and mechanism of PGN4.9 nanoadjuvant for reprogramming M2- like TAMs into M1 phenotype via pH-gated regulation of lysosomal function
Experiment 1: Development and Characterization of pH-Gated Nanoparticles (PGNs)
Primary Technique: The primary technique utilized in this study was the synthesis and characterization of pH-gated nanoparticles (PGNs) designed for selective targeting of M2-like tumor-associated macrophages (TAMs).
Key Steps:
- Synthesis: A library of amphiphilic copolymers was synthesized using an atom transfer radical polymerization (ATRP) method, incorporating various monomers to achieve pH sensitivity.
- Characterization: The copolymers were characterized via 1H-NMR spectroscopy and gel permeation chromatography to confirm successful synthesis and to determine molecular weight and polydispersity.
- pH Transition Point (pHt) Evaluation: The pKa values were measured through titration in acidic buffers, establishing a range of pHt from 4.5 to 5.5 for different PGNs.
Data Collection and Analysis: The pHt and stability of the synthesized PGNs were assessed using fluorescence spectroscopy. The hydrodynamic diameter and zeta potential were analyzed with a Zetasizer Nano ZSP.
Result: The synthesized PGNs exhibited a sharp pH response (ΔpHON/OFF ~ 0.2–0.3) and maintained colloidal stability in physiological conditions, with PGN4.9 identified as the most effective nanoparticle for targeting M2-like TAMs based on its transition point of 4.9.
Novel Aspects: The study introduced a novel approach to engineer PGNs with precise pH tunability, allowing for targeted delivery specifically to M2-like TAMs, which is a significant advancement over traditional nano-delivery systems that lack such selectivity.
Experiment 2: In Vitro Activation of PGN4.9 in M2-like Macrophages
Primary Technique: The primary technique employed was fluorescence microscopy to evaluate the activation of PGN4.9 within M2-like macrophages.
Key Steps:
- Cell Culture: Bone marrow-derived macrophages (BMDMs) were polarized to M2-like phenotype using interleukin-4 (IL-4) for 24 hours.
- Nanoparticle Treatment: M2-like BMDMs were treated with PGN4.9 nanoparticles for 2 hours at 37 °C.
- Fluorescent Labeling: Cells were stained with LysoTracker Red to visualize lysosomes and then imaged using a confocal microscope.
Data Collection and Analysis: Fluorescence intensity was measured using ImageJ software to quantify the activation of PGN4.9 in M2-like macrophages, focusing on the ratio of “OFF” to “ON” fluorescence signals.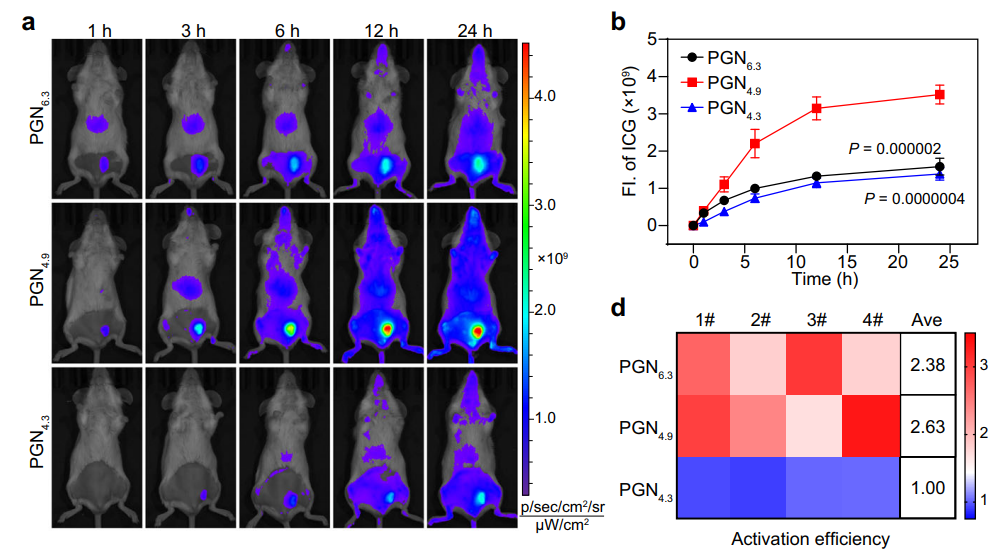
Figure 2. PGN4.9 selectively targets to M2-like TAMs in vivo. a Representative fluorescence images and b fluorescence intensity of tumour sites at different timepoints after intravenous injection of ICG-conjugated PGNs (20 mg kg−1 ) in 4T1 tumour-bearing mice (n = 4 mice; two-way ANOVA followed by Tukey’s multiple comparisons test)
Result: The results indicated that PGN4.9 was selectively activated in M2-like macrophages, turning the “OFF” signal “ON” in their acidic lysosomes while remaining inactive in M0- and M1-like macrophages.
Novel Aspects: This experiment demonstrated the selective activation of PGN4.9 in M2-like macrophages, showcasing a targeted delivery mechanism that enhances the effectiveness of immunotherapy compared to conventional delivery systems that lack specificity.
Experiment 3: In Vivo Imaging of PGN4.9 Accumulation in Tumors
Primary Technique: The primary technique used was in vivo fluorescence imaging to assess the accumulation of PGN4.9 in tumor tissues.
Key Steps:
- Animal Model: 4T1 tumor-bearing BALB/c mice were established by subcutaneously injecting 4T1 cells.
- Administration: Cy5-conjugated PGN4.9 nanoparticles were intravenously injected into the mice at a dosage of 20 mg/kg.
- Imaging: Fluorescence images were captured at pre-defined time points using an IVIS Spectrum imaging system.
Data Collection and Analysis: The fluorescence intensity from tumor tissues was quantified and normalized against control groups. Data analysis was performed using ImageJ to calculate the selectivity index of PGN4.9 in targeting M2-like TAMs.
Result: The in vivo imaging revealed significant accumulation of PGN4.9 in tumor tissues, with a notable preference for M2-like TAMs over M0- and M1-like macrophages, and minimal accumulation in healthy tissues.
Novel Aspects: This experiment introduced a method for real-time monitoring of nanoparticle accumulation in tumors, providing insights into the selective targeting capabilities of PGN4.9, which enhances the potential for safer and more effective cancer therapies.
Experiment 4: Assessment of Immunomodulatory Effects of PGN4.9
Primary Technique: The primary technique was flow cytometry to evaluate the immunomodulatory effects of PGN4.9 on macrophage phenotype repolarization.
Key Steps:
- Treatment: M2-like BMDMs were treated with PGN4.9 nanoadjuvant for 24 hours, along with control groups (PBS, NPGN).
- Surface Marker Staining: Cells were stained with antibodies against CD86 (M1 marker) and CD206 (M2 marker) for flow cytometry analysis.
- Cytokine Assay: Supernatants were collected for ELISA to measure pro-inflammatory cytokines, such as IL-12.
Data Collection and Analysis: Flow cytometry was used to quantify the expression levels of CD86 and CD206, while ELISA results were analyzed statistically to compare cytokine production across treatment groups.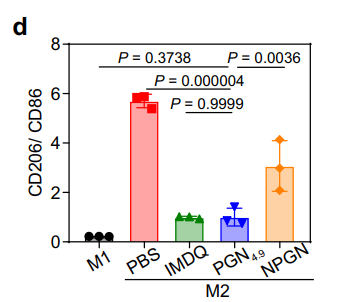
Figure 3. Ratio of CD206 to CD86 expression in BMDMs lysate by flow cytometry (n = 3 experiments).
Result: PGN4.9 treatment significantly increased the expression of M1 markers (CD86) and IL-12 production, while decreasing M2 markers (CD206), indicating effective repolarization of M2-like macrophages to M1-like phenotype.
Novel Aspects: This experiment highlighted the ability of PGN4.9 to effectively repolarize M2-like TAMs, enhancing their antigen presentation and immune activation capabilities, which is more efficient than traditional methods that do not provide such targeted reprogramming.
Experiment 5: Evaluation of Antigen Presentation and T Cell Activation
Primary Technique: The primary technique utilized was an antigen presentation assay in conjunction with T cell activation assessment.
Key Steps:
- Antigen Treatment: PGN4.9-treated M2-like BMDMs were pulsed with ovalbumin (OVA) peptides for 24 hours.
- Flow Cytometry Analysis: Cells were stained for MHC Class I and costimulatory markers (CD80, CD86) and analyzed by flow cytometry.
- T Cell Activation Assay: Splenocytes from OVA-immunized mice were co-cultured with treated macrophages to assess the activation of CD8+ T cells.
Data Collection and Analysis: The proportion of SIINFEKL-H-2Kb+ cells and activated CD8+ T cells were quantified using flow cytometry, with results analyzed statistically to determine significance.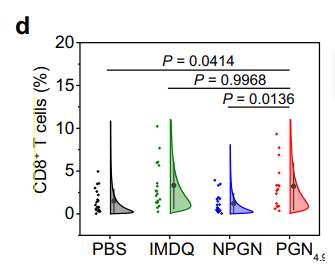
Figure 4. CD8+ T cells from images in panel (a) (n = 16 regions for PGN4.9 group; n = 18 regions for IMDQ group; n = 20 regions for other groups)
Result: The results demonstrated that PGN4.9-treated macrophages enhanced the cross-presentation of OVA peptides, leading to a substantial increase in the activation of CD8+ T cells compared to control groups.
Novel Aspects: This experiment provided evidence for the enhanced antigen cross-presentation capabilities of PGN4.9-treated macrophages, showcasing a novel approach to improving T cell responses in cancer immunotherapy that surpasses traditional methods of antigen delivery.
Conclusion
The successful development of the pH-gated nanoparticle (PGN) delivery system was achieved through a comprehensive approach that combined advanced nanotechnology with a deep understanding of the tumor microenvironment. By engineering nanoparticles that specifically target the acidic lysosomal environment of M2-like tumor-associated macrophages (TAMs), the research team was able to selectively modulate these immune cells and promote their reprogramming into a more effective M1-like phenotype. This strategic targeting enhances the macrophages’ ability to present antigens and elicit robust anti-tumor immune responses while minimizing off-target effects on healthy tissues.
The highlights of the study include the demonstration of PGN4.9’s ability to significantly reduce lysosomal acidity in M2-like TAMs, resulting in improved antigen cross-presentation and activation of CD8+ T cells. Furthermore, the research provided compelling evidence for the enhanced therapeutic efficacy of PGN4.9 in vivo, leading to sustained tumor regression in mouse models. Overall, this innovative drug delivery strategy represents a promising advancement in cancer immunotherapy, offering potential pathways to improve patient outcomes by effectively reprogramming immune cells in the tumor microenvironment.
Reference
Tang, Mingmei, et al. “pH-gated nanoparticles selectively regulate lysosomal function of tumour-associated macrophages for cancer immunotherapy.” Nature Communications, vol. 14, no. 5888, 2023, doi:10.1038/s41467-023-41592-0.
