Editor: Nina
Scientists develop a multifunctional nanoparticle system that selectively depletes NADPH in tumor cells while enhancing anti-PD-L1 therapy and reprogramming the tumor microenvironment to improve treatment outcomes for triple-negative breast cancer.
Key Preview
Research Question
The study investigates how to enhance the efficacy of anti-PD-L1 immunotherapy for triple-negative breast cancer (TNBC) patients, particularly those with low PD-L1 expression and immunosuppressive tumor microenvironments (TMEs).
Research Design and Strategy
The researchers designed a multifunctional nanomedicine that combines low-dose radiotherapy with a specific NADPH-depleting agent to improve the immunotherapy response against TNBC.
Method
The study involved synthesizing a “drug-like” copolymer, creating nanoparticles (BMS202@HZP NPs) that target hypoxic tumor cells, and evaluating their effects in vitro and in vivo.
Key Results
The BMS202@HZP NPs successfully depleted NADPH in tumor cells, enhanced PD-L1 expression, and reprogrammed the TME to alleviate immunosuppression, resulting in significantly improved anti-tumor efficacy.
Significance of the Research
This innovative approach may provide a promising strategy to overcome the limitations of current anti-PD-L1 therapies in TNBC, potentially leading to better outcomes for patients.
Introduction
Triple-negative breast cancer (TNBC) is a particularly aggressive subtype of breast cancer characterized by the absence of estrogen and progesterone receptors, as well as a lack of overexpression of the human epidermal growth factor receptor 2 (HER2). This subtype accounts for approximately 15-20% of all breast cancer cases and is associated with a higher likelihood of metastasis and poorer prognosis compared to other breast cancer subtypes. The five-year survival rate for late-stage TNBC patients is alarmingly low, hovering around 14%. The limited treatment options available for TNBC often result in a reliance on traditional chemotherapy, which, while effective in some cases, is hindered by significant toxicity and a lack of specificity for cancer cells.
Common strategies for drug delivery in traditional treatments involve systemic administration of chemotherapeutic agents. These agents circulate throughout the body, targeting both cancerous and healthy cells indiscriminately. While this approach can reduce tumor size, it often leads to severe side effects due to damage to normal tissues. Moreover, the efficacy of such treatments is often limited by the tumor microenvironment (TME), which can be immunosuppressive and hinder the infiltration of therapeutic agents into the tumor.
Current approaches to treating TNBC face distinct challenges, including low expression levels of prognostic markers such as PD-L1 in many tumors, which limits the effectiveness of immunotherapy and targeted therapies. Additionally, the immunosuppressive nature of the TME further complicates treatment outcomes, resulting in a substantial number of patients not responding to standard therapies. This dilemma underscores the pressing need for innovative strategies that can enhance therapeutic efficacy while mitigating adverse effects.
To address these challenges, recent advancements in nanomedicine have introduced novel drug delivery systems that offer enhanced specificity and efficacy. One such innovative strategy involves the development of multifunctional nanoparticles that can selectively deliver therapeutic agents to tumor cells while simultaneously modulating the TME. These nanoparticles are designed to exploit the unique characteristics of tumor cells, such as hypoxia and overexpression of specific receptors, allowing for targeted release of drugs that can enhance the immune response and improve treatment outcomes. By utilizing a combination of radiotherapy and targeted drug delivery, this approach aims to not only increase the therapeutic response in TNBC patients but also to convert “cold” tumors into “hot” tumors, thereby improving the efficacy of anti-PD-L1 therapies.
Research Team and Aim
The research team was led by Dr. Ying Wang, along with co-researchers Di Gao, Lin Jin, and others, from Xi’an Jiaotong University and affiliated institutions. This study was conducted in 2022 and culminated in the publication of their findings in the paper titled “NADPH Selective Depletion Nanomedicine-Mediated Radio-Immunometabolism Regulation for Strengthening Anti-PD-L1 Therapy against TNBC,” which appeared in the journal Advanced Science.
The primary aim of the research, as articulated by Dr. Wang, was to develop a nanomedicine that not only enhances the effectiveness of anti-PD-L1 therapy but also mitigates the immunosuppressive effects of the tumor microenvironment in triple-negative breast cancer (TNBC) patients. Specifically, the study sought to create a multifunctional drug delivery system that could selectively deplete NADPH in tumor cells while maintaining NADPH levels in immune cells, ultimately improving the therapeutic response to combined low-dose radiotherapy and immunotherapy.
Experimental Process
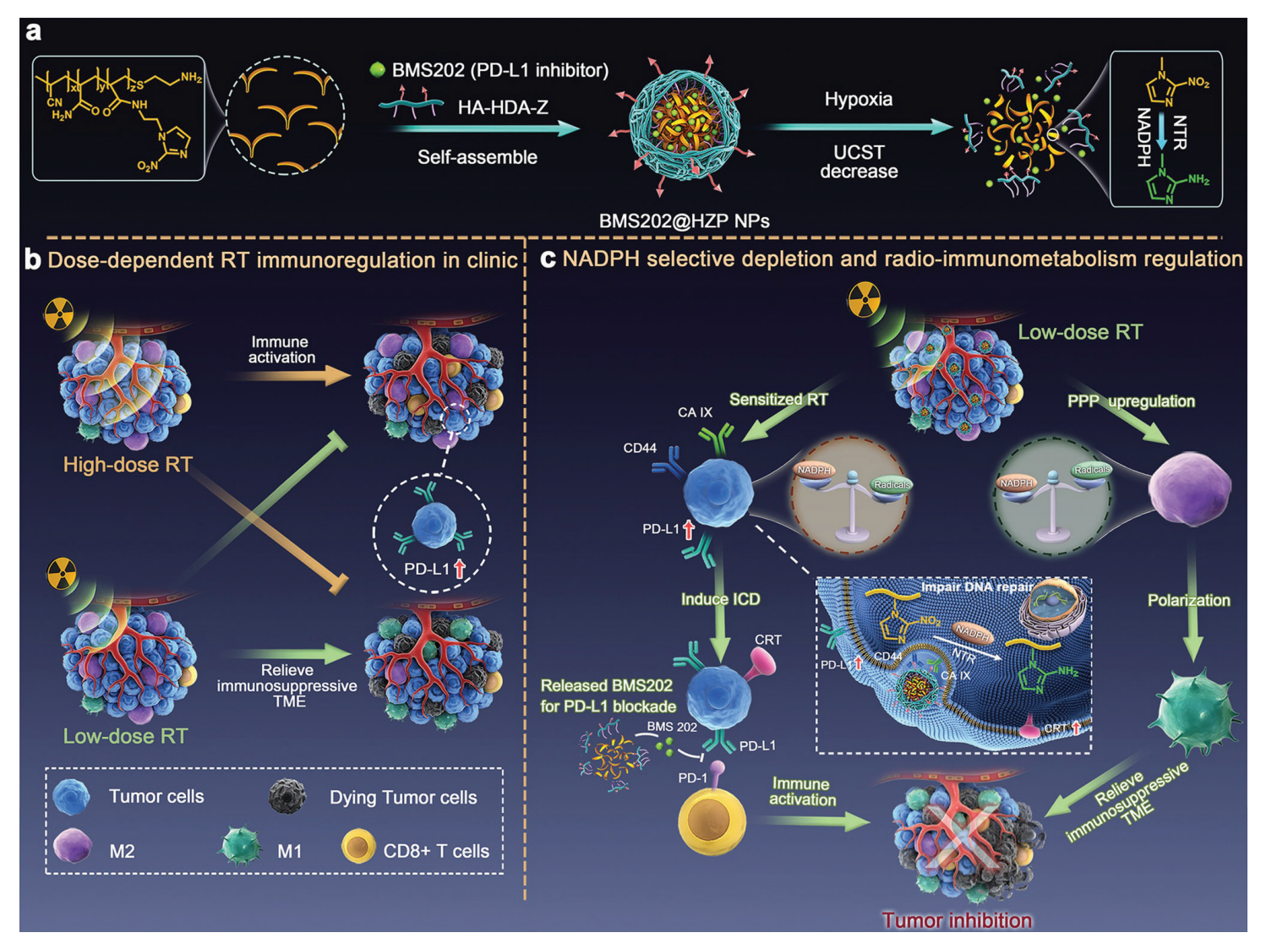
Figure 1.Production and application of the BMS202@HZP NPs. a) Schematic illustration of design and preparation of BMS202@HZP NPs. b) Dosedependent RT immunoregulation in clinic. c) Mechanism of BMS202@HZP NPs-mediated NADPH selective depletion and radio-immunometabolism regulation for synergistically anti-PDL1 therapy against TNBC.
Experiment 1: Synthesis of BMS202@HZP Nanoparticles
Primary Technique: The primary technique employed in this experiment was the synthesis of multifunctional nanoparticles using a radical polymerization method.
Key Steps:
- The copolymer poly(acrylonitrile-co-acrylamide-co-N-(2-(2-nitro-1H-imidazol-1-yl)ethyl)acrylamide (PAAN) was synthesized through radical polymerization involving the monomers acrylonitrile (AN), acrylamide (AAm), and N-(2-(2-nitro-1H-imidazol-1-yl)ethyl)acrylamide (NIEAAm).
- The synthesized PAAN copolymer was then mixed with hyaluronic acid-modified hexadecylamine-acetazolamide (HA-HDA-Z) to form the nanoparticles (HZP NPs) encapsulating BMS202, an antagonist of PD-1/PD-L1 interaction.
- The resulting nanoparticles were characterized for size, zeta potential, and encapsulation efficiency.
Data Collection and Analysis:
Data on nanoparticle size and zeta potential were collected using dynamic light scattering (DLS) and electrophoretic mobility analysis, respectively. Encapsulation efficiency was determined using UV-Vis spectroscopy to measure the absorbance of BMS202 in the supernatant after centrifugation.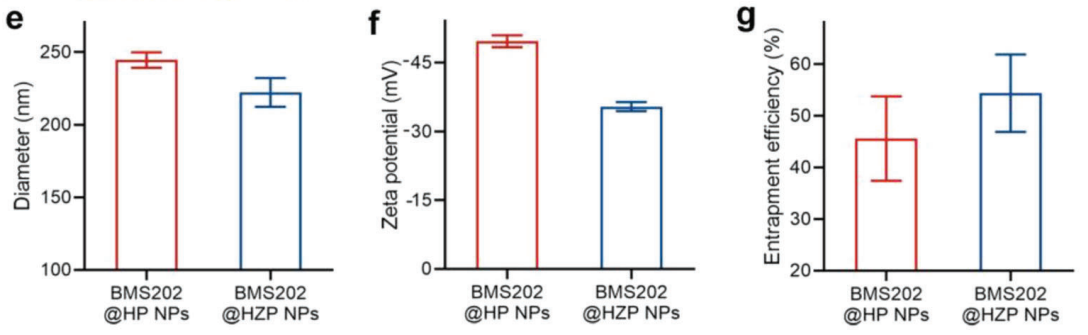
Figure 2. . e–g) Characterization of different NPs, including e) diameter, f) zeta potential, and g) encapsulation efficiency.
Result:
The synthesized BMS202@HZP NPs displayed an average size of 222.2 nm and a zeta potential of -35.4 mV, indicating good stability in solution. The encapsulation efficiency of BMS202 was found to be 54.5%.
Novel Aspect:
This synthesis method introduces a dual-targeting mechanism via HA and CA IX, enhancing the accumulation of nanoparticles in hypoxic TNBC cells. This approach improves upon traditional nano-delivery systems by enabling controlled drug release based on the tumor microenvironment, thus increasing therapeutic efficacy.
Experiment 2: In Vitro Evaluation of Antitumor Efficacy
Primary Technique: The primary technique utilized in this experiment was in vitro cytotoxicity assays using the MTT assay and colony formation assay.
Key Steps:
- 4T1 TNBC cells were cultured and treated with varying concentrations of BMS202@HZP NPs and irradiated with low-dose radiation (2 Gy).
- The MTT assay was performed to assess cell viability post-treatment, where absorbance was measured at 570 nm.
- For the colony formation assay, treated cells were seeded in a 6-well plate and allowed to grow for two weeks, after which colonies were fixed and stained for visualization.
Data Collection and Analysis:
Cell viability data from the MTT assay were statistically analyzed using GraphPad Prism software, determining IC50 values for different treatments. The colony formation assay results were quantified by counting the colonies and calculating the survival fraction.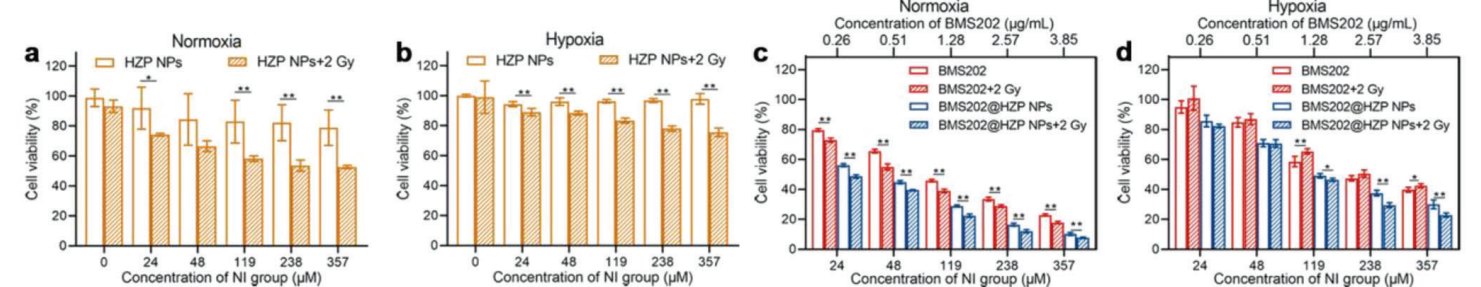
Figure 3. a–d) Cytotoxicity evaluation of blank a,b) HZP NPs, c,d) BMS202 and BMS202@HZP NPs with or without LDRT (2 Gy) against 4T1 cells at various NI-equivalent concentrations under hypoxia and normoxia (n = 5).
Result:
BMS202@HZP NPs significantly reduced cell viability in 4T1 cells, with IC50 values indicating synergistic effects when combined with low-dose radiation. The colony formation assay revealed only 30.8% and 57.1% of colonies remained viable after treatment under normoxic and hypoxic conditions, respectively.
Novel Aspect:
The study demonstrates a strategic combination of radiotherapy and a targeted nanoparticle system. This dual approach enhances the cytotoxicity of conventional treatments by ensuring that the drug is released specifically within the tumor environment, overcoming the limitations of traditional systemic therapies.
Experiment 3: In Vivo Biodistribution Studies
Primary Technique: The primary technique used in this experiment was fluorescence imaging to assess the biodistribution of BMS202@HZP NPs in mice.
Key Steps:
- Mice bearing subcutaneous 4T1 tumors were injected with DiR-loaded HZP NPs and subjected to X-ray radiation (2 Gy) to enhance nanoparticle accumulation in tumors.
- Fluorescence imaging was conducted at various time points (1, 6, 12, and 24 hours post-injection) to monitor the distribution of nanoparticles in vivo.
Data Collection and Analysis:
Fluorescence intensity in tumor tissues and major organs was quantified using an imaging system, and the data were analyzed to compare the accumulation of DiR in tumors pre- and post-radiation.
Result:
The fluorescence imaging revealed a 2.5-fold increase in nanoparticle accumulation in tumors after radiation treatment compared to controls, indicating successful targeting.
Novel Aspect:
This experiment highlights the synergistic role of low-dose radiation in enhancing the delivery of nanoparticles to tumors, a novel approach that shows promise in improving the efficiency of cancer therapies compared to conventional passive targeting methods.
Experiment 4: Mechanistic Studies of Immune Response
Primary Technique: The primary technique employed was transcriptome analysis through RNA sequencing to evaluate changes in immune-related gene expression.
Key Steps:
- Mice bearing 4T1 tumors were treated with BMS202@HZP NPs combined with LDRT, and tumor tissues were harvested post-treatment for RNA extraction.
- RNA sequencing was performed to identify differentially expressed genes related to immune response.
Data Collection and Analysis:
Bioinformatics tools were used to analyze RNA sequencing data, including gene ontology and pathway enrichment analysis to identify significant changes in gene expression profiles.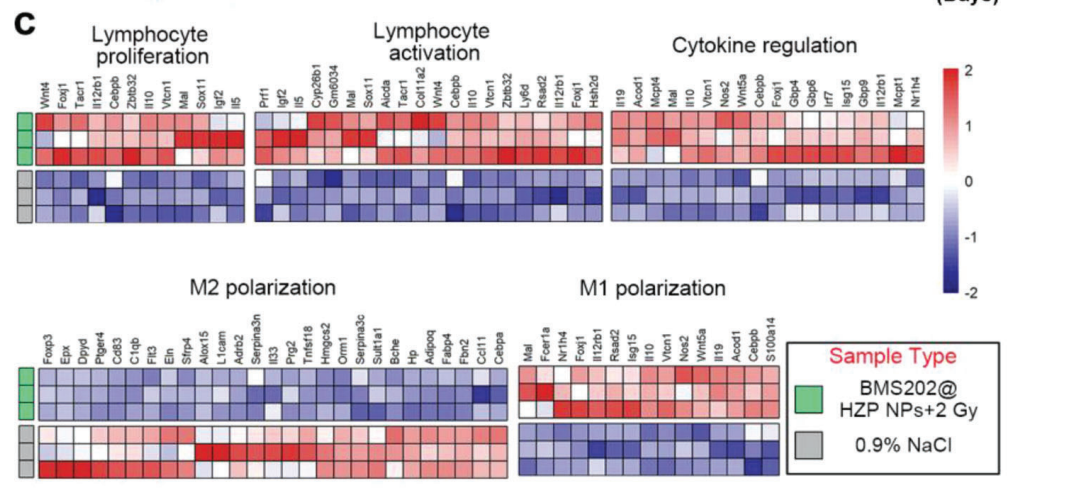
Figure 4. c) Heatmap of differentially expressed genes associated with immune response of 4T1 tumors following the indicated treatments on day 10 (fold change ≥ 2, FDR-adjusted p-value < 0.05).
Result:
The analysis revealed significant upregulation of immune activation genes and downregulation of immunosuppressive pathways in tumors treated with BMS202@HZP NPs plus LDRT.
Novel Aspect:
This study introduces a comprehensive mechanistic understanding of how the novel nanoparticle system enhances immune responses in the tumor microenvironment, shifting the immunological landscape from a suppressive to an active state, a significant advancement over conventional therapies.
Conclusion
The successful development of the BMS202@HZP nanoparticle drug delivery system was achieved through a multifaceted approach that combines the principles of nanomedicine, targeted drug delivery, and immunometabolic regulation. By synthesizing a novel “drug-like” copolymer that selectively depletes NADPH in tumor cells while maintaining NADPH levels in immune cells, the researchers created a system capable of enhancing the effectiveness of anti-PD-L1 therapy against triple-negative breast cancer (TNBC). This dual targeting strategy not only improved the accumulation of the nanoparticles in hypoxic tumor environments but also facilitated the controlled release of the PD-L1 antagonist, BMS202, thereby amplifying its therapeutic effects.
The highlights of the study include the demonstration that BMS202@HZP NPs can effectively convert “cold” tumors into “hot” tumors, thereby improving the immunological response against TNBC. The study also provided compelling evidence of the nanoparticles’ ability to reprogram the tumor microenvironment, alleviate immunosuppression, and significantly enhance the anti-tumor efficacy when combined with low-dose radiotherapy. Collectively, these findings underscore the potential of this innovative drug delivery strategy to revolutionize treatment approaches for TNBC and possibly other cancers with similar challenges.
Reference
Wang, Ying, et al. “NADPH Selective Depletion Nanomedicine-Mediated Radio-Immunometabolism Regulation for Strengthening Anti-PD-L1 Therapy against TNBC.” Advanced Science, vol. 10, no. 2, 2023, Article 2203788. doi:10.1002/advs.202203788.
