Novel Oral mRNA Vaccine Delivery System Could Improve Vaccine Accessibility
In an important development, researchers from EvoBiotech and the University of Turin have introduced a new method for delivering mRNA vaccines orally. This approach, detailed in a recent study, utilizes plant-derived extracellular vesicles (EVs) from orange juice (Citrus sinensis) to encapsulate mRNA vaccines. This presents a potential alternative to traditional injection-based systems, addressing key challenges in global vaccine distribution, particularly in terms of accessibility, cost, and the need for refrigeration.
While mRNA vaccines have shown great success in combating COVID-19, their cold storage requirements and injection-based delivery methods pose logistical barriers, particularly in resource-limited regions. This research proposes a solution by developing an oral mRNA vaccine that can be stored at room temperature and administered without the need for syringes or medical professionals, thus increasing accessibility.
Building on previous advances in EV technology, which has shown promise in drug and vaccine delivery, the researchers demonstrated that mRNA encapsulated in plant-derived EVs remains stable at room temperature for up to 12 months. Notably, when administered to rats through oral capsules, this mRNA formulation successfully triggered a strong immune response, including the production of IgM, IgG, and IgA antibodies—key components for immunity.
The system was tested using EVs derived from orange juice, which were loaded with mRNA encoding the S1 protein subunit of the SARS-CoV-2 virus. After lyophilization and encapsulation in gastro-resistant capsules, the mRNA remained intact and functional. Rats that received the oral vaccine exhibited a strong humoral immune response, suggesting that the vaccine was both stable and effective, capable of inducing both local and systemic immunity.
This delivery method represents a significant advancement in vaccine technology. The use of plant-derived EVs as carriers for mRNA is not only scalable but also non-toxic, making it a cost-effective option for large-scale vaccine production. The researchers also note the potential of this system for other vaccines and therapeutic agents, offering promise for more efficient vaccination efforts globally.
Contributions and Key Findings:
Development of Oral mRNA Vaccine: This study is one of the first to develop a method for delivering mRNA vaccines orally using plant-derived EVs, specifically from orange juice. The system allows the vaccine to be stored at room temperature, addressing key storage and distribution challenges for mRNA vaccines.
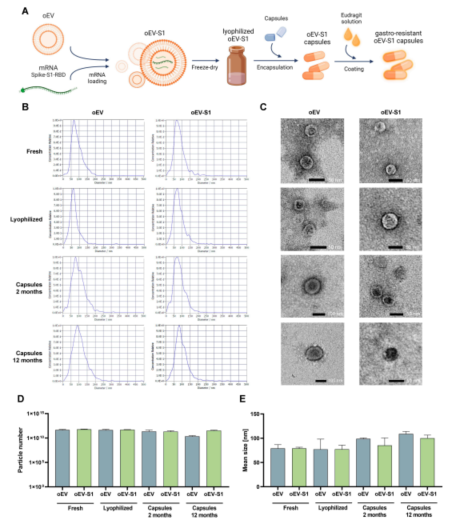
Figure 1: oEV characterization and formulation.
Stability and Functionality: The researchers demonstrated that mRNA loaded into plant-derived EVs remained stable at room temperature for up to 12 months. The mRNA retained its functionality even after lyophilization and encapsulation in gastro-resistant capsules, which protect the mRNA from degradation in the stomach and release it in the intestines where it can trigger an immune response.
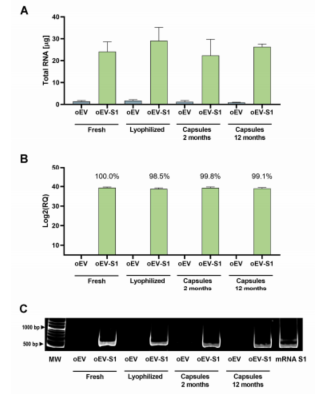
Figure 2: mRNA maintaining over time.
Immune Response Induction: When administered orally to rats, the vaccine induced a robust immune response, including the production of IgM, IgG, and IgA antibodies. IgA is particularly important for mucosal immunity, providing a defense at the entry points of the virus. The immune response also demonstrated neutralization activity, blocking the binding of the S1 protein to the ACE2 receptor.
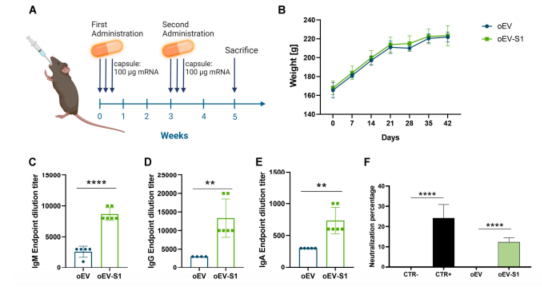
Figure 3: Rat vaccination with oral capsules.
Non-Toxicity and Safety: The study confirmed that the vaccine formulation did not cause toxicity in rats, as evidenced by normal animal behavior and the absence of morphological changes in tissue samples. This suggests that the oral vaccine is safe for use without the need for medical supervision.
Scalable and Cost-Effective: The use of plant-derived EVs as a delivery vehicle is both scalable and non-toxic, making it a promising option for mass production of vaccines at a low cost. This system could also be adapted for the delivery of other vaccines and therapeutic agents, expanding its potential applications in global health efforts.
Potential for Global Vaccine Accessibility: The oral vaccine delivery system, coupled with the ability to store the vaccine at room temperature, offers significant advantages in terms of accessibility, especially in areas with limited healthcare infrastructure. This approach could streamline vaccine distribution and administration, making it easier and more affordable to deploy vaccines worldwide.
Conclusion:
The findings of this study represent a promising step forward in the development of more accessible and cost-effective vaccines. By using plant-derived EVs to deliver mRNA vaccines orally, this research offers a scalable solution to many of the logistical challenges currently facing global vaccine distribution. While further research is needed to optimize the vaccine formulation and fully understand its potential in humans, the implications of this work could greatly improve the speed and scale of vaccination campaigns worldwide.
Reference
Pomatto, Margherita A. C., et al. “Oral Delivery of mRNA Vaccine by Plant-Derived Extracellular Vesicle Carriers.” Cells, vol. 12, no. 14, 2023, p. 1826. MDPI, https://doi.org/10.3390/cells12141826.
