Advances in Cancer Immunotherapy: mRNA-LNP Delivery of EpCAM-CD3 Bispecific Antibodies Shows Promise for Colorectal Cancer Treatment
In a recent study, researchers have unveiled significant progress in cancer immunotherapy. By utilizing mRNA-Lipid Nanoparticles (LNPs) to deliver bispecific antibodies targeting EpCAM (Epithelial Cell Adhesion Molecule) and CD3, they have demonstrated a new approach for treating colorectal cancer. This novel delivery system showed potential for blocking tumor growth, suggesting a path toward more effective treatments in the future. The study emphasizes the possibility of providing a more targeted and less toxic alternative to current therapies, particularly as resistance to conventional cancer treatments continues to grow.
Colorectal cancer remains one of the most prevalent cancers globally, presenting a significant challenge to researchers and clinicians. Despite the development of treatments such as monoclonal antibodies, vaccines, and immunotherapies, there remains a pressing need for therapies that can more effectively target the disease. This research raises the question: Could the mRNA-LNP delivery system hold the key to better, more efficient cancer treatments?
Contribution and Key Findings: A Step Forward in Colorectal Cancer Research
This study builds on previous research focused on colorectal cancer therapies, particularly those targeting EpCAM, a protein often overexpressed in a range of cancers, including colorectal cancer. EpCAM has been identified as a promising target for cancer immunotherapy, and bispecific antibodies targeting both EpCAM and CD3 have already shown potential in preclinical models. However, this new research advances this approach by utilizing mRNA-LNPs to directly deliver these antibodies to the tumor site.
Key findings from the study include:
Engineering of Bispecific Antibodies: Researchers engineered and tested three different designs of humanized EpCAM-CD3 bispecific antibodies, including CrossMab knob-in-hole (KIH), BITE, and the EpCAM ScFv-CD3 ScFv-human Fc construct. These antibodies demonstrated high specificity, effectively binding to EpCAM-positive cells while avoiding EpCAM-negative cells. This specificity is crucial in minimizing off-target effects, which is a common issue in cancer therapies.
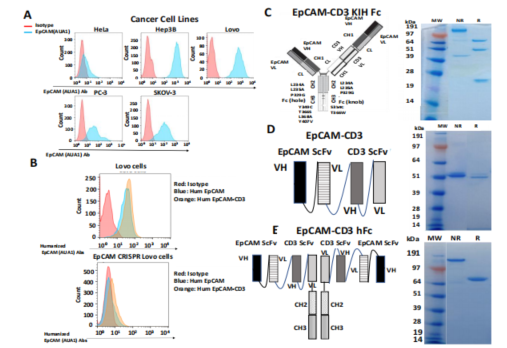
Figure 1: Binding characteristics of three differently designed EpCAM-CD3 antibodies.
In Vitro and In Vivo Efficacy: The bispecific antibodies demonstrated substantial efficacy in vitro, with a dose-dependent ability to kill EpCAM-positive cells and induce the secretion of IFN-gamma from T cells, further boosting the immune response. The in vivo experiments, particularly those involving OVCAR-5 xenograft tumors in mice, showed that the delivery of these antibodies significantly inhibited tumor growth. Notably, the treatment was more effective when T cells were injected alongside the antibodies, highlighting the importance of immune system activation.
mRNA-LNP Delivery Technology: The study employed a novel approach by using mRNA-LNPs to deliver the EpCAM-CD3 bispecific antibodies. This method offers enhanced stability, lower production costs, and ease of scalability compared to traditional protein-based therapies. Furthermore, it eliminates the need for complex protein manufacturing, making the process more efficient and cost-effective.
Safety Profile: The safety of the mRNA-LNP delivery system was rigorously tested. The study showed no increase in toxicology markers (e.g., ALT, AST, amylase, and LDH) in the serum of treated mice, suggesting that the treatment does not result in significant toxicity. This is an encouraging sign for the potential of mRNA-LNP-based therapies to offer safer alternatives to conventional treatments.
Methodology: A New Approach to Cancer Treatment
The study utilized mRNA-LNPs to deliver the EpCAM-CD3 bispecific antibodies, a technology that has gained attention due to its use in COVID-19 vaccines by Moderna and Pfizer. The methodology involved several critical steps:
mRNA-LNP Generation: Researchers synthesized mRNA encoding the EpCAM-CD3 bispecific antibodies and embedded it into lipid nanoparticles (LNPs). This process was optimized to ensure that the mRNA was effectively delivered to tumor cells in a stable and controlled manner.
In Vitro Validation: In vitro tests demonstrated that the antibodies, when delivered via mRNA-LNPs, effectively bound to EpCAM-positive cancer cells and induced T-cell-mediated killing. The combination of the antibody and T cells also led to significant secretion of IFN-gamma, a key immune molecule that enhances the body’s ability to fight tumors.
In Vivo Validation: The treatment was tested in mice with OVCAR-5 xenograft tumors. The results showed a significant reduction in tumor size and weight, confirming the efficacy of the therapy. Importantly, the treatment did not result in increased levels of toxicology markers in the serum, indicating a favorable safety profile.
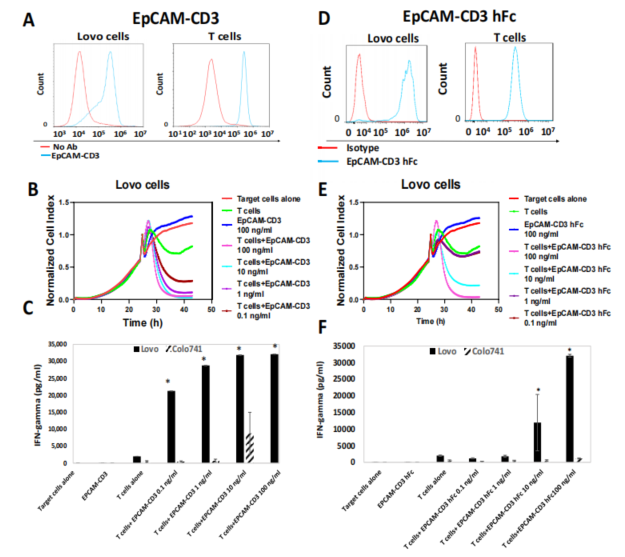
Figure 2: Binding of EpCAM-CD3 hFc antibody to EpCAM-positive Lovo cells in the presence of T cells.
Conclusion: A Potential New Path for Cancer Immunotherapy
The findings from this study suggest a promising new approach to cancer immunotherapy, particularly for colorectal cancer. By combining mRNA-LNP delivery technology with bispecific antibodies, researchers have developed a treatment modality that is highly targeted, potentially less toxic, and more cost-effective than traditional antibody therapies. As the next steps involve further clinical trials and optimization of the mRNA-LNP platform, this approach may be applicable to treating other solid tumors and cancer types.
The future of cancer treatment may depend on the continued development of this advanced technology, which holds promise for better-targeted therapies and improved outcomes for cancer patients.
Reference
Golubovskaya, Vita, et al. “mRNA-Lipid Nanoparticle (LNP) Delivery of Humanized EpCAM-CD3 Bispecific Antibody Significantly Blocks Colorectal Cancer Tumor Growth.” Cancers, vol. 15, no. 10, 2023, p. 2860. MDPI, https://doi.org/10.3390/cancers15102860.
