Editor: Nina
Scientists construct cancer cell membrane-coated gambogic acid nanoparticles to enhance anti-tumor immunity and improve therapeutic efficacy in colorectal cancer treatment.
Key Preview
- Research Question
This study investigates whether cancer cell membrane-coated gambogic acid nanoparticles (CCM-PLGA/GA NPs) can enhance anticancer vaccination by activating dendritic cells and improving the immune response against colorectal cancer (CRC). - Research Design and Strategy
The researchers utilized a two-step emulsification method to create poly (lactic-co-glycolic acid) (PLGA) nanoparticles loaded with gambogic acid (GA). They then coated these nanoparticles with CT26 colon cancer cell membranes to form CCM-PLGA/GA NPs, aiming to enhance tumor targeting and immune activation. - Method
The method involved synthesizing nanoparticles, confirming their stability and targeting ability, and assessing their toxicity. In vitro and in vivo studies were conducted to evaluate the efficacy of the nano-vaccine in activating dendritic cells and inducing a favorable immune microenvironment. - Key Results
The study found that CCM-PLGA/GA NPs exhibited low toxicity, high tumor-targeting ability, and significant activation of dendritic cells. In vivo experiments showed improved anti-tumor efficacy, especially when combined with anti-PD-1 monoclonal antibodies. - Significance of the Research
This research highlights a novel strategy for CRC immunotherapy using a nano-vaccine that not only directly targets tumors but also modulates the immune microenvironment, potentially enhancing treatment outcomes for CRC patients.
Introduction
Colorectal cancer (CRC) is one of the most prevalent and lethal malignancies worldwide, ranking as the third most diagnosed cancer and the second leading cause of cancer-related deaths. The complexity of CRC is underscored by its multifactorial etiology, which includes genetic, environmental, and lifestyle factors. As the disease progresses, it often becomes resistant to standard therapeutic interventions, leading to a poor prognosis for patients diagnosed with advanced stages. Traditional treatment strategies for CRC typically involve a combination of surgical resection, chemotherapy, and targeted therapies, with the aim of reducing tumor burden and preventing recurrence.
In conventional chemotherapy, drugs are administered systemically to target rapidly dividing cancer cells. However, this approach comes with significant limitations, including off-target effects, non-specific toxicity to healthy tissues, and the challenge of achieving therapeutic concentrations at the tumor site. These challenges often result in suboptimal treatment outcomes, with many patients experiencing debilitating side effects and reduced quality of life. Additionally, the development of drug resistance poses a serious hurdle, further complicating treatment regimens and leading to cancer recurrence.
To address these significant challenges, innovative drug delivery strategies are being explored. One promising approach is the use of nanotechnology in drug delivery systems, which allows for the encapsulation of therapeutic agents in nanoparticles. This method enhances the targeting and bioavailability of drugs while minimizing systemic toxicity. Among these advanced strategies, the development of biomimetic nanoparticles coated with cancer cell membranes has emerged as a groundbreaking avenue. By leveraging the unique properties of cancer cell membranes, such as their ability to evade the immune system and homotypically target tumor cells, researchers aim to create a more effective and safer means of delivering anticancer therapies. This study investigates the potential of cancer cell membrane-coated gambogic acid nanoparticles as a novel nano-vaccine to improve anti-tumor immunity in colorectal cancer, representing a significant advancement in the quest for effective cancer treatment.
Research Team and Aim
The research team for this study was led by Dr. Fengli Huang, who is affiliated with the Department of Oncology at Nanjing Drum Tower Hospital, Clinical College of Nanjing University of Chinese Medicine. The research was conducted in 2023 and culminated in the publication of their findings in the paper titled “Cancer Cell Membrane-Coated Gambogic Acid Nanoparticles for Effective Anticancer Vaccination by Activating Dendritic Cells.” This work was published in the International Journal of Nanomedicine.
The aim of the research, as articulated by Dr. Huang, was to construct a novel nano-vaccine that improves the anti-tumor immune response in colorectal cancer (CRC) by utilizing gambogic acid (GA) as an adjuvant, alongside cancer cell membranes to enhance tumor targeting and immune activation. The goal was to provide a new strategy for the immunotherapy of CRC that could potentially lead to better therapeutic efficacy and reduced side effects for patients.
Experimental Process
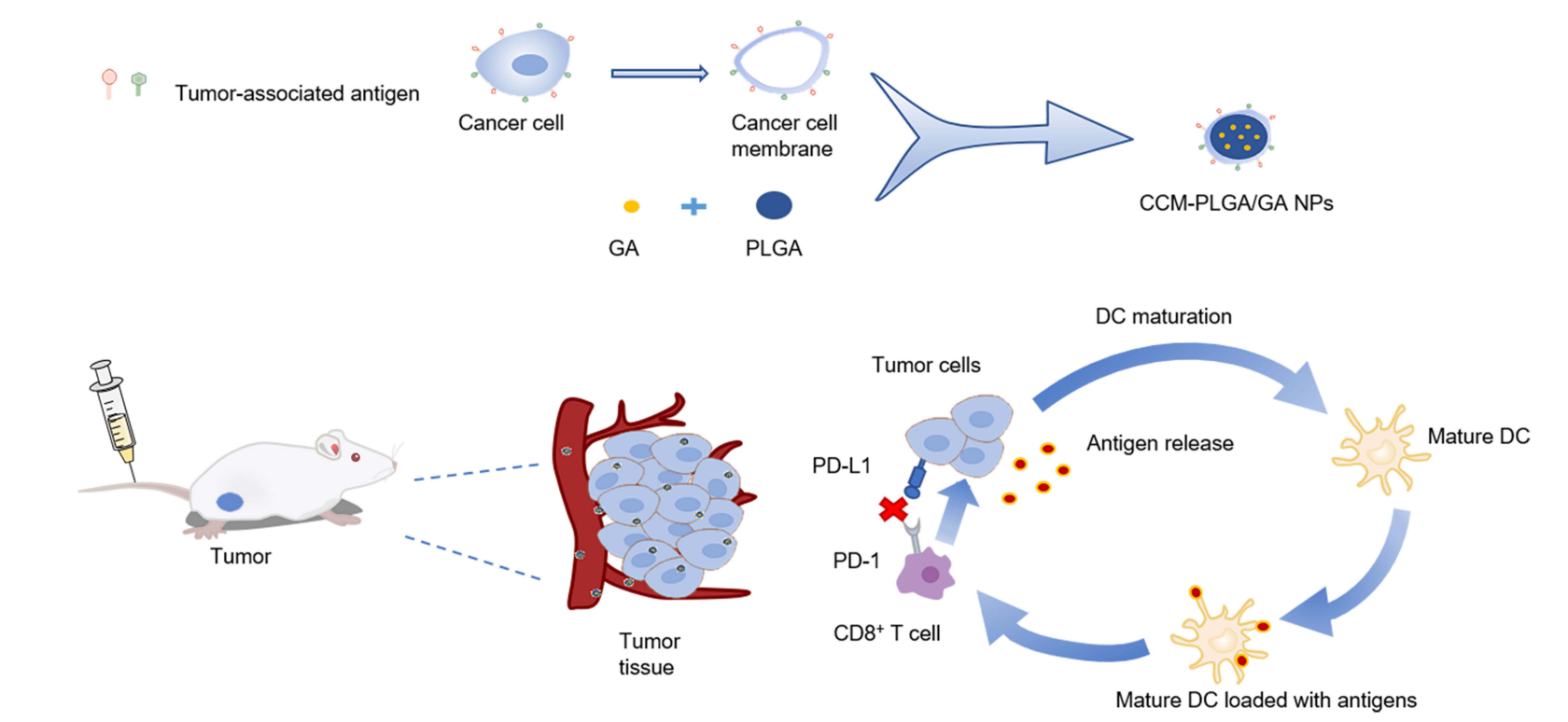
Experiment 1: Synthesis of CCM-PLGA/GA Nanoparticles
Primary Technique:
The main method employed in this study for synthesizing the nanoparticles was a two-step emulsification technique.
Key Steps:
- Preparation of PLGA/GA Nanoparticles:
- Dissolve poly (lactic-co-glycolic acid) (PLGA) and gambogic acid (GA) in a suitable organic solvent.
- Employ a two-step emulsification method to generate the first emulsion (oil-in-water) by mixing the organic phase with an aqueous phase containing polyvinyl alcohol (PVA).
- Stir the emulsion to facilitate solvent evaporation, leading to the formation of PLGA/GA nanoparticles.
- Coating with CT26 Cancer Cell Membrane:
- Harvest CT26 colon cancer cells and subject them to freeze-thaw cycles to extract the cancer cell membrane.
- Extrude the PLGA/GA nanoparticles through a small extruder with the CT26 cancer cell membrane to create the CCM-PLGA/GA nanoparticles.
Data Collection and Analysis:
The size and polydispersity index (PDI) of the synthesized nanoparticles were measured using Dynamic Light Scattering (DLS). Transmission Electron Microscopy (TEM) was utilized to visualize the morphology and confirm the successful coating of the nanoparticles.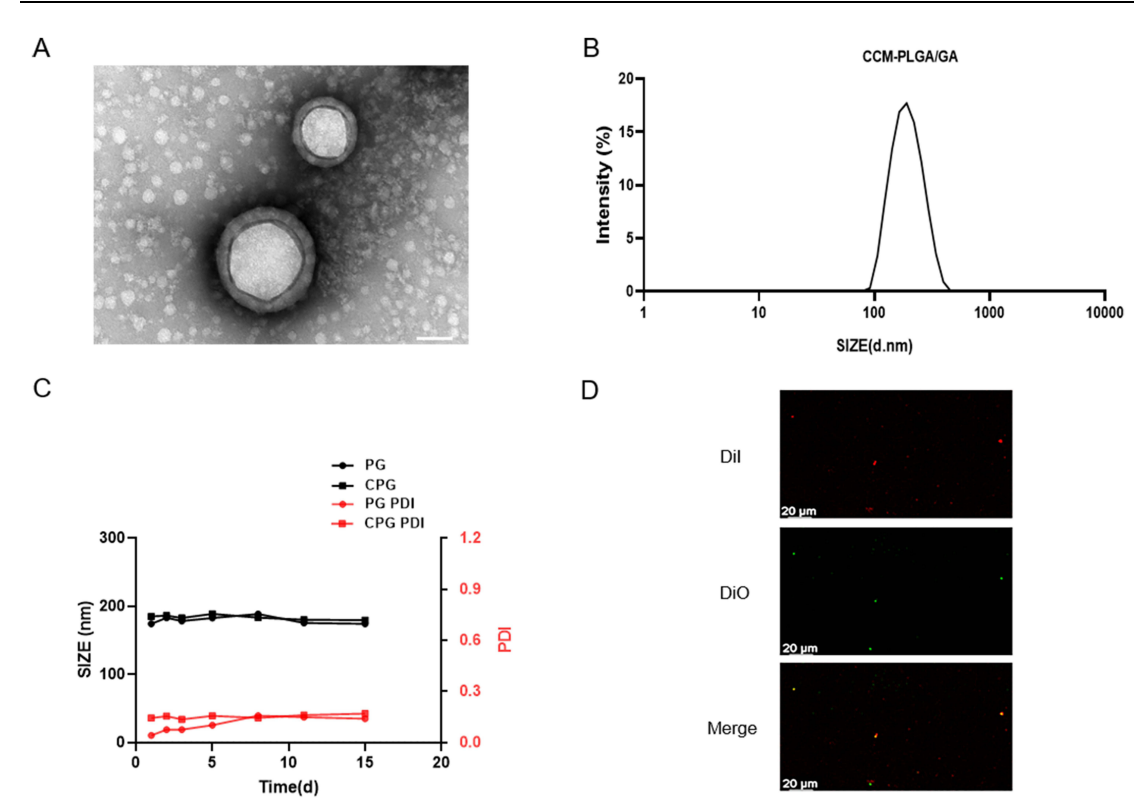
Figure 1. Characterization of CCM-PLGA/GA NPs. (A) TEM images of CCM-PLGA /GA NPs. (The scale bar is 50 nm). (B) Size distribution of CCM-PLGA/GA NPs determined via DLS. © Size and PDI of PG and CPG NPs in PBS over 14 days. (D) Confocal images of the colocalization between DiO-labeled CCM and DiI-labeled NPs. (The scale bar is 20 μm).
Result:
The synthesized CCM-PLGA/GA nanoparticles exhibited an average diameter of approximately 182 nm with a stable PDI, confirming the successful synthesis and coating processes. The encapsulation efficiency of GA in the nanoparticles was found to be 85.5%.
Novel Aspect:
This experiment introduced a novel biomimetic approach by utilizing cancer cell membranes for coating the nanoparticles, enhancing tumor targeting capabilities compared to traditional delivery systems that lack specificity. The dual functionality of targeting and drug delivery offers a more effective therapeutic strategy.
Experiment 2: In Vitro Cytotoxicity Assay
Primary Technique:
The CCK8 assay was employed to assess the cytotoxic effects of the CCM-PLGA/GA nanoparticles on CT26 colon cancer cells.
Key Steps:
- Cell Culture:
- Culture CT26 cells in a 96-well plate overnight to allow for cell attachment.
- Treatment Application:
- Expose the cells to various concentrations of CCM-PLGA/GA nanoparticles and free GA for 24 hours.
- CCK8 Reagent Addition:
- Add 10 µL of CCK8 reagent to each well and incubate for an additional 40 minutes to allow for color development.
Data Collection and Analysis:
The absorbance at 450 nm was measured using a spectrophotometer to quantify cell viability. Data were analyzed using statistical methods to determine significant differences between treated and control groups.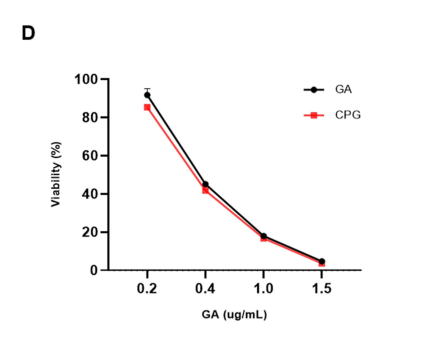
Figure 2. In vitro cytotoxicity of CCM-PLGA/GA NPs in comparison to free GA on CT26 cells after 24 hours of incubation (n=4, P >0.05).
Result:
The results indicated that both free GA and the CCM-PLGA/GA nanoparticles exhibited significant cytotoxic effects on CT26 cells. However, there were no statistically significant differences in cell viability between the two treatments, suggesting that the encapsulation did not compromise GA’s efficacy.
Novel Aspect:
The use of the CCK8 assay in conjunction with the novel CCM-PLGA/GA nanoparticles demonstrated an effective method for evaluating the therapeutic potential of nanoparticle formulations, providing a more accurate assessment of their cytotoxic properties compared to traditional methods.
Experiment 3: In Vivo Biodistribution Study
Primary Technique:
Near-Infrared (NIR) fluorescence imaging was utilized to assess the biodistribution of the nanoparticles in a CT26 tumor-bearing mouse model.
Key Steps:
- Mouse Model Preparation:
- Subcutaneously inject 5 × 10^5 CT26 cells into the right flank of BALB/c mice. Allow tumor growth until the average tumor volume reached approximately 100 mm³.
- NP Administration:
- Randomly assign mice to receive either PLGA/DiR or CCM-PLGA/DiR nanoparticles via intravenous injection.
- Fluorescence Imaging:
- Perform NIR fluorescence imaging at designated time points (e.g., 24 hours post-injection) to visualize nanoparticle accumulation in the tumor and other organs.
Data Collection and Analysis:
Quantitative analysis of fluorescence intensity in the tumor and organs was performed using imaging software, allowing for comparisons between groups.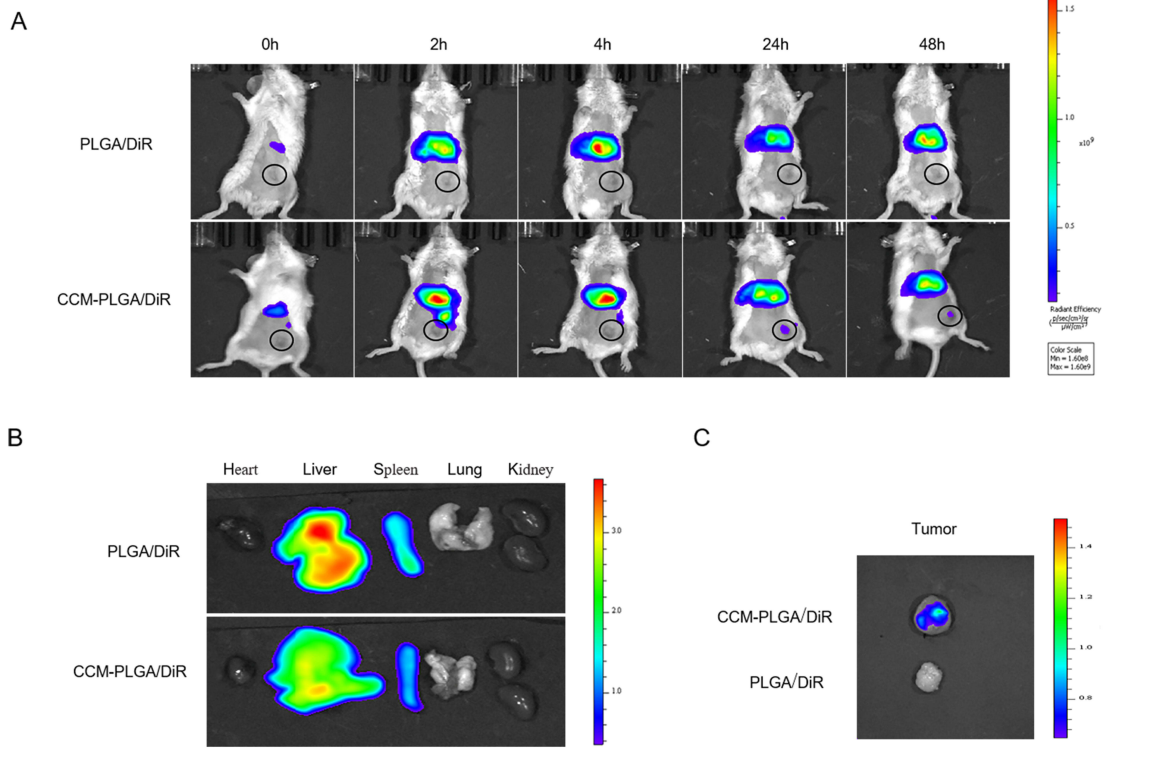
Figure 3. In Vivo Biodistribution and targeting ability. (A) Representative real-time NIR fluorescence imaging of CT26 tumor-bearing mice following intravenous injection of PLGA/DiR and CCM-PLGA/DiR NPs. The tumor of each mouse was circled. (B and C) Ex vivo NIR images of the various organs and tumor at 24 hours postadministration with two different NPs
Result:
CCM-PLGA/DiR nanoparticles showed significantly higher fluorescence intensity at the tumor site compared to PLGA/DiR nanoparticles, indicating enhanced tumor targeting capability.
Novel Aspect:
This study introduced a non-invasive imaging technique to evaluate the biodistribution of the novel nanoparticles, showcasing their potential for improved targeting of tumors compared to standard delivery systems.
Experiment 4: Immune Response Evaluation
Primary Technique:
Flow cytometry was employed to evaluate the immune response induced by the CCM-PLGA/GA nanoparticles in the tumor microenvironment.
Key Steps:
- Treatment of Tumor-Bearing Mice:
- Administer saline, PLGA/GA, or CCM-PLGA/GA nanoparticles to different groups of tumor-bearing mice once a week for two weeks.
- Tissue Collection:
- After the final treatment, sacrifice mice and collect spleens and tumor tissues for analysis.
- Flow Cytometry Staining:
- Prepare single-cell suspensions from spleens and tumors, then stain with specific antibodies targeting CD11c, CD80, CD86, and CD8+ T cells.
Data Collection and Analysis:
Flow cytometric analysis was performed to quantify the populations of mature dendritic cells (mDCs) and CD8+ T cells, with statistical analysis conducted to determine significance.
Result:
The results indicated a significant increase in the populations of mDCs and CD8+ T cells in the CCM-PLGA/GA group compared to controls, suggesting effective immune activation.
Novel Aspect:
This experiment demonstrated the unique capacity of the CCM-PLGA/GA nanoparticles to enhance immune responses against tumors, highlighting their potential as a novel immunotherapeutic strategy that differs from traditional therapies that often fail to elicit strong immune activation.
Experiment 5: In Vivo Antitumor Efficacy
Primary Technique:
A combination therapy approach was used to evaluate the antitumor efficacy of CCM-PLGA/GA nanoparticles in conjunction with anti-PD-1 monoclonal antibodies.
Key Steps:
- Establishment of Tumor Model:
- Implant CT26 cells into BALB/c mice to establish the tumor model, allowing the tumors to grow to a set volume.
- Treatment Groups:
- Randomly assign treatment groups receiving saline, PLGA/GA, anti-PD-1, CCM-PLGA/GA, and a combination of CCM-PLGA/GA with anti-PD-1.
- Monitoring Tumor Growth:
- Measure tumor volume every few days using calipers, and calculate the average tumor volume for each group.
Data Collection and Analysis:
Statistical analysis was performed on tumor growth data, using ANOVA to assess the significance of differences between treatment groups.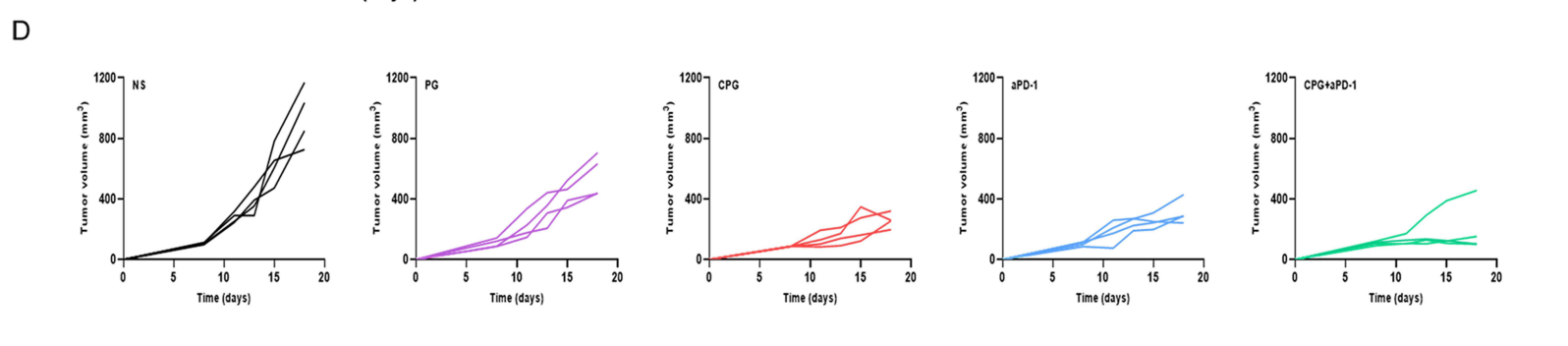
Figure 4. Tumor growth curves in five groups of each mouse (n=4).
Result:
The combination therapy group (CCM-PLGA/GA + anti-PD-1) exhibited the most significant tumor suppression compared to other groups, with tumor volumes remaining below 170 mm³ by the end of the study.
Novel Aspect:
This experiment provided compelling evidence for the synergistic effects of combining the novel nano-vaccine with immune checkpoint blockade, representing a promising strategy for enhancing antitumor efficacy in CRC beyond traditional monotherapy approaches.
Conclusion
The successful development of the cancer cell membrane-coated gambogic acid nanoparticles (CCM-PLGA/GA NPs) as a novel drug delivery system was achieved through a meticulous synthesis process that involved the encapsulation of gambogic acid in poly (lactic-co-glycolic acid) (PLGA) nanoparticles, followed by the innovative coating of these nanoparticles with cancer cell membranes. This biomimetic approach not only enhanced the targeting capabilities of the nanoparticles but also allowed for better immune evasion, thereby improving the bioavailability of the therapeutic agent at the tumor site.
The highlights of this study include the demonstration of low biological toxicity and high tumor-targeting ability of the CCM-PLGA/GA NPs. Furthermore, the study revealed significant activation of dendritic cells, leading to a favorable immune microenvironment that promotes anti-tumor immunity. Notably, the combination therapy of the nano-vaccine with anti-PD-1 monoclonal antibodies exhibited enhanced anti-tumor efficacy, showcasing the potential of this innovative delivery system for improving treatment outcomes in colorectal cancer patients. Overall, this research presents a promising new strategy for immunotherapy that may pave the way for more effective and safer cancer treatments.
Reference
Huang, Fengli, et al. “Cancer Cell Membrane-Coated Gambogic Acid Nanoparticles for Effective Anticancer Vaccination by Activating Dendritic Cells.” International Journal of Nanomedicine, vol. 18, 2023, pp. 2261-2273. doi:10.2147/IJN.S408521.
