Editor: Nina
Scientists develop human serum albumin-based nanoparticles for the stable and sustained delivery of basic fibroblast growth factor to enhance wound healing and tissue regeneration.
Key Preview
Research Question
Can human serum albumin-based nanoparticles effectively deliver basic fibroblast growth factor (bFGF) to enhance wound healing and tissue regeneration?
Research Design and Strategy
The study employs a combination of in vitro and in vivo experiments to evaluate the effectiveness of bFGF-loaded human serum albumin nanoparticles (HSA-bFGF NPs) in promoting wound healing.
Method
The researchers created HSA-bFGF NPs through a desolvation and crosslinking process, followed by extensive characterization and testing in human dermal fibroblasts and rat wound models.
Key Results
HSA-bFGF NPs demonstrated enhanced stability and sustained release of bFGF, leading to improved fibroblast proliferation, migration, and reduced scar formation in wound healing.
Significance of the Research
This research addresses the limitations of bFGF’s short half-life and instability, offering a promising delivery system for regenerative medicine.
Introduction
Wound healing is a complex biological process that can be significantly impaired due to various factors such as chronic diseases, infections, or physical trauma. When the skin barrier is disrupted, the body initiates a series of overlapping phases, including hemostasis, inflammation, proliferation, and remodeling, to restore tissue integrity. However, delayed or improper healing can lead to complications such as chronic wounds, infections, and excessive scarring, which can severely impact a patient’s quality of life. Conditions such as diabetes and certain vascular disorders can further exacerbate these issues, making effective wound management essential.
Traditional strategies for drug delivery in wound healing often involve the use of topical applications or systemic administration of growth factors and cytokines, such as basic fibroblast growth factor (bFGF). These methods aim to enhance cellular proliferation, migration, and angiogenesis, thereby promoting tissue regeneration. However, a significant challenge with these conventional approaches is the inherent instability and short half-life of many therapeutic proteins, including bFGF. These factors result in rapid degradation and loss of efficacy when administered, necessitating frequent dosing and limiting their overall therapeutic potential.
Moreover, the burst release often associated with traditional drug delivery systems can lead to suboptimal concentrations of the drug at the target site over time. This not only hampers the desired biological response but can also increase the risk of adverse effects, including inflammation or scarring due to excessive local concentrations of the drug.
To address these challenges, innovative drug delivery strategies have emerged, focusing on the development of nanoparticle-based systems that encapsulate therapeutic agents. One such approach involves the use of human serum albumin (HSA)-based nanoparticles for the secure delivery of bFGF. This method capitalizes on the biocompatibility and stability of HSA, allowing for controlled and sustained release of the growth factor at the wound site. By enhancing the stability and bioavailability of bFGF, this innovative system seeks to improve the efficacy of wound healing therapies, reduce the frequency of dosing, and ultimately enhance patient outcomes in regenerative medicine.
Research Team and Aim
The research was conducted by a multidisciplinary team of scientists, led by Dr. Hee Ho Park, at Hanyang University and Ulsan National Institute of Science and Technology. The study took place in 2023 and was published in the Journal of Nanobiotechnology under the title “Secured delivery of basic fibroblast growth factor using human serum albumin-based protein nanoparticles for enhanced wound healing and regeneration.”
The aim of the research, as articulated by Dr. Park, was to “develop a stable, effective delivery system for bFGF that addresses its limitations in clinical settings, thereby enhancing wound healing and tissue regeneration.” This objective underscores the commitment of the research team to innovate in the field of regenerative medicine through advanced drug delivery methods.
Experimental Process
Primary Technique
The primary technique utilized in this study is the preparation and characterization of human serum albumin-based nanoparticles (HSA-bFGF NPs) through a desolvation method. This approach facilitates the secure loading of basic fibroblast growth factor (bFGF) onto the albumin nanoparticles, allowing for enhanced stability, sustained release, and improved therapeutic efficacy in wound healing and tissue regeneration.
Key Steps
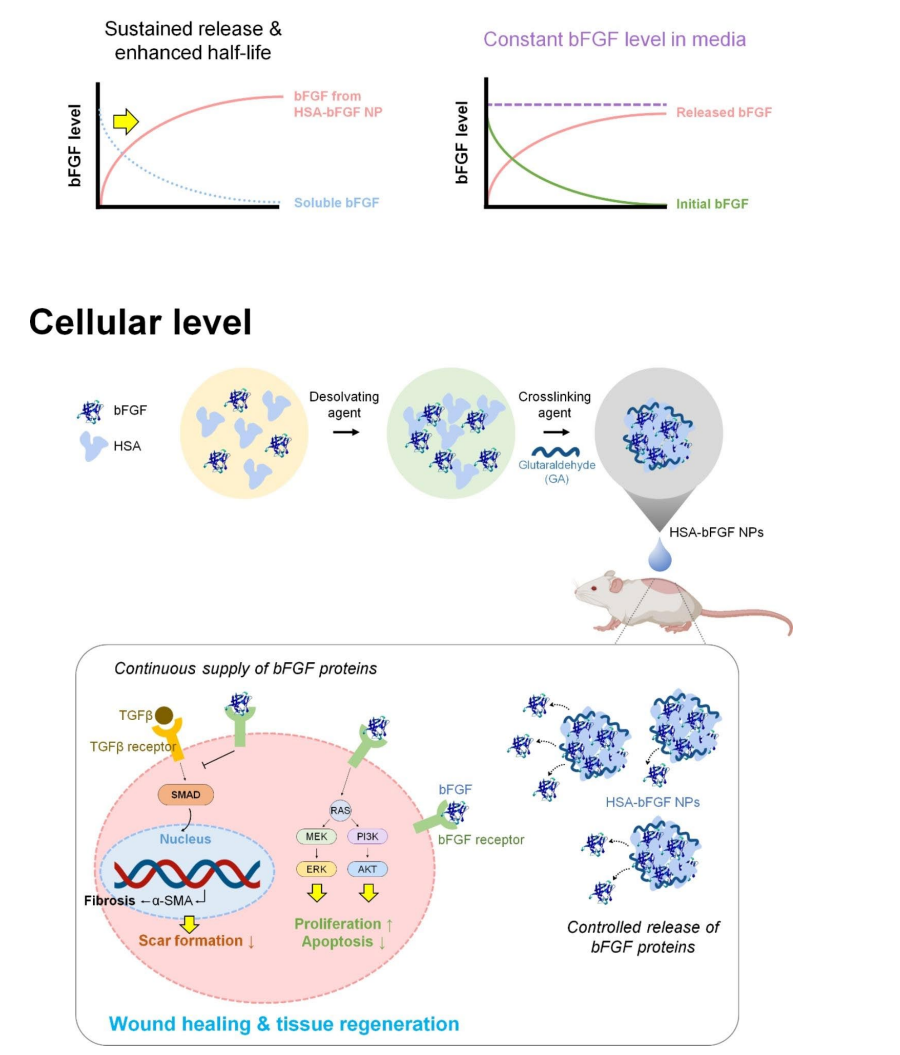
Figure 1. Schematic illustrations depicting cellular delivery of bFGF, followed by wound healing cascades of HSA-bFGF NPs. Intact bFGF has short half-life both in culture media and tissue microenvironment, thus secured crosslinking of bFGF with HSA to form a nanoparticulate is essentially advantageous for long-term supplement of biologically active bFGF in tissue regeneration.
- Production of bFGF Protein: The recombinant bFGF protein was produced using a genetically modified expression vector (pET-28a/FGF2-G3) in E. coli. The bacterial cultures were grown in LB medium containing 100 µg/ml ampicillin and induced with 1 mM IPTG at either 37°C for 4 hours (fast induction) or 30°C for 16 hours (slow induction). Following induction, cells were lysed through ultrasonication, and the bFGF protein was purified using a HisTrap HP column. The final concentration of purified bFGF was analyzed via SDS-PAGE and Western blotting.
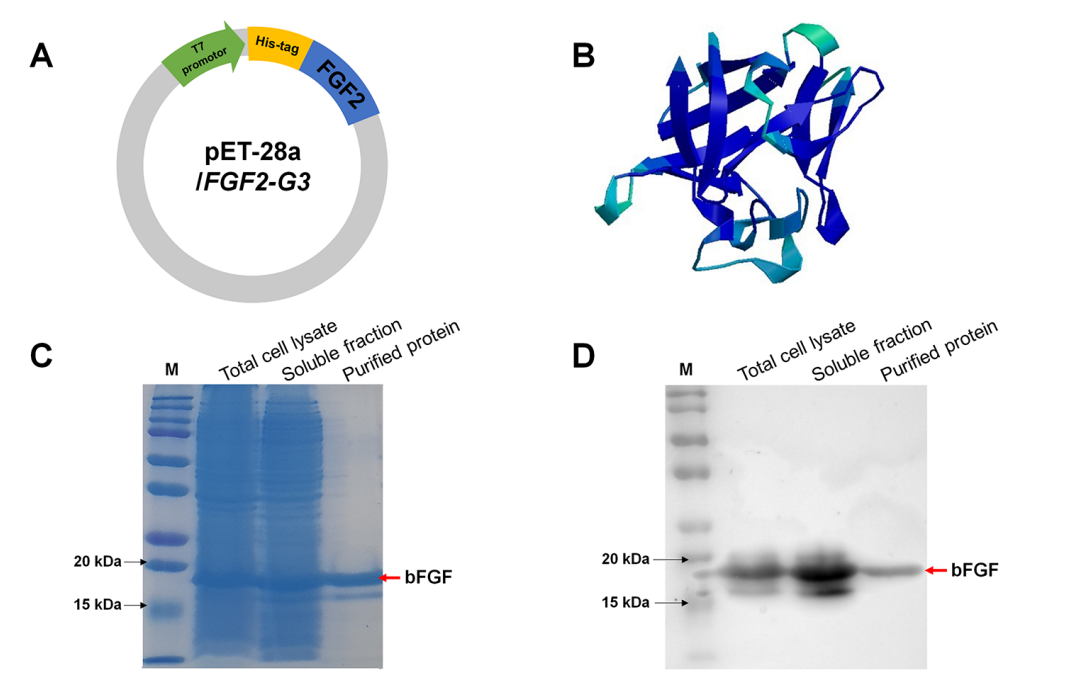
Figure 2. (A) Construction of E. coli expression vector, pET-28a/FGF2-G3. (B) Three-dimensional representation structure of bFGF protein. © SDS-PAGE analysis of the puriffed recombinant bFGF protein. M means marker. Red arrow indicates bFGF protein. (D) Western blot analysis of bFGF protein produced from E. coli. M means marker. Red arrow indicates bFGF protein.
- Preparation of HSA-bFGF NPs: Human serum albumin (HSA) was mixed with the purified bFGF protein in Tris-HCl buffer (pH 8.0) under constant stirring. Ethanol was added dropwise to induce desolvation, promoting the formation of protein aggregates. Crosslinking was achieved by adding varying amounts of glutaraldehyde (GA) to stabilize the nanoparticles. The resulting HSA-bFGF NPs were purified via centrifugation and resuspended in phosphate-buffered saline (PBS) for further analysis.
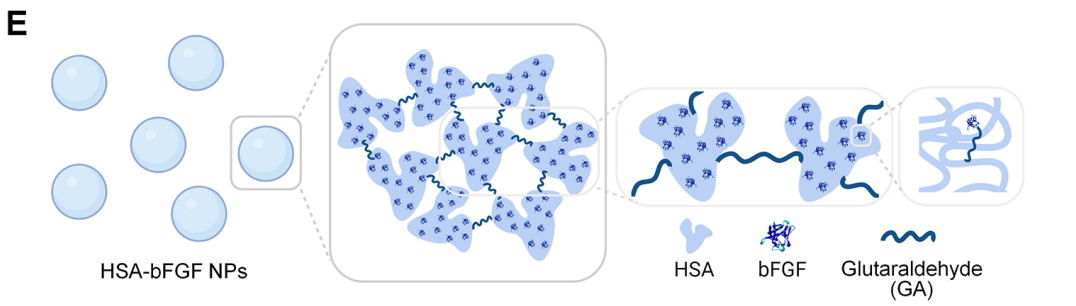
Figure 3. Schematic illustrations demonstrating formation of HSA-bFGF NPs via crosslinking between HSA and bFGF mediated by GA
- In Vitro Characterization: The size, polydispersity index (PI), and zeta-potential of the prepared HSA-bFGF NPs were measured using an electrophoretic light scattering (ELS) spectrophotometer. Scanning electron microscopy (SEM) was employed to visualize the morphology of the nanoparticles. The in vitro release profile of bFGF from the nanoparticles was assessed through enzyme-linked immunosorbent assay (ELISA) over a 12-day period, with measurements taken daily.
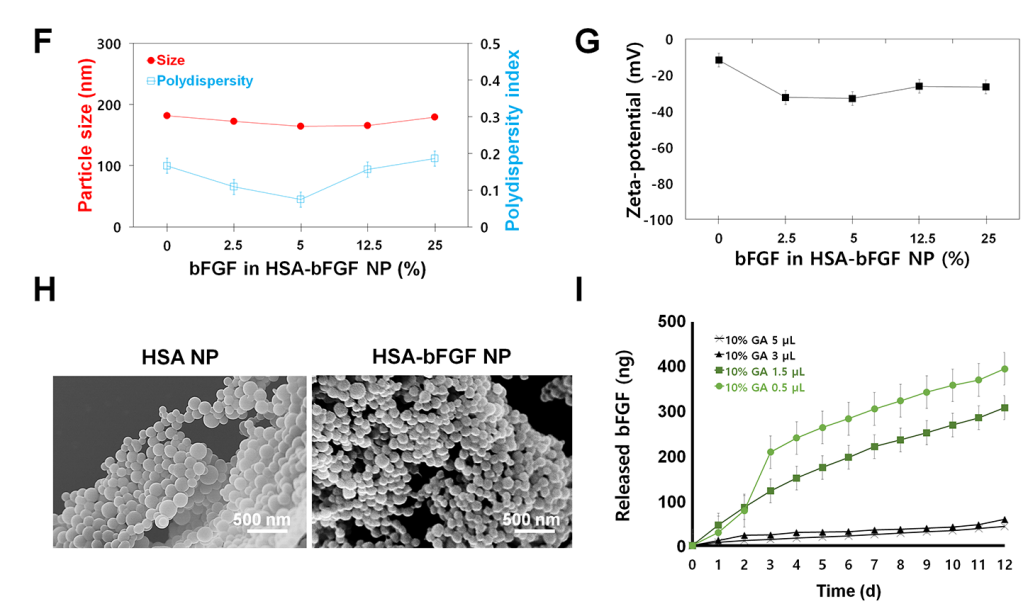
Figure 4. (F) Nanoparticle size and PI depending on the bFGF contents (wt%) in HSA-bFGF NPs. (G) Zeta-potential changes of the HSA-bFGF NPs. (H) SEM images of HSA-bFGF NPs containing 0 or 25 wt% of bFGF protein, respectively. Scale bars, 500 nm. (I) In vitro release proffle of bFGF from HSA-bFGF NPs depending upon the amount of added crosslinker, GA
- Cell Proliferation and Migration Assays: Human dermal fibroblasts (HDFs) were treated with HSA-bFGF NPs, HSA NPs (control), commercial bFGF, and produced bFGF to evaluate cell proliferation using the CCK-8 assay and BrdU incorporation. Migration activity was assessed using a wound closure assay, where a scratch was made in a confluent HDF culture and the area of closure was measured over time.
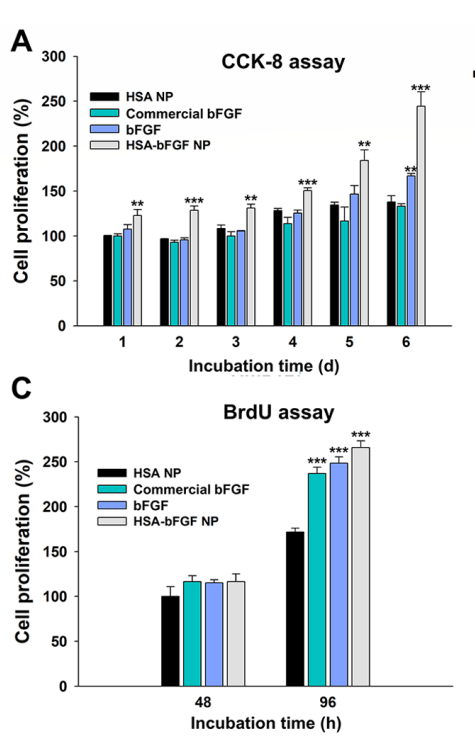
Figure 5. (A) CCK-8 assay of HDFs for cell proliferation analysis.© BrdU assay of HDFs for DNA synthesis analysis.
- In Vivo Wound Healing Model: Excisional wounds (~1 cm in diameter) were created on the backs of Sprague Dawley rats, which were divided into four treatment groups: non-treated (control), HSA NPs, soluble bFGF, and HSA-bFGF NPs. Each treatment was administered directly to the wound site. Wound closure was monitored over 14 days, and tissue samples were collected for histological analysis, including H&E staining and immunofluorescence for blood vessel formation.
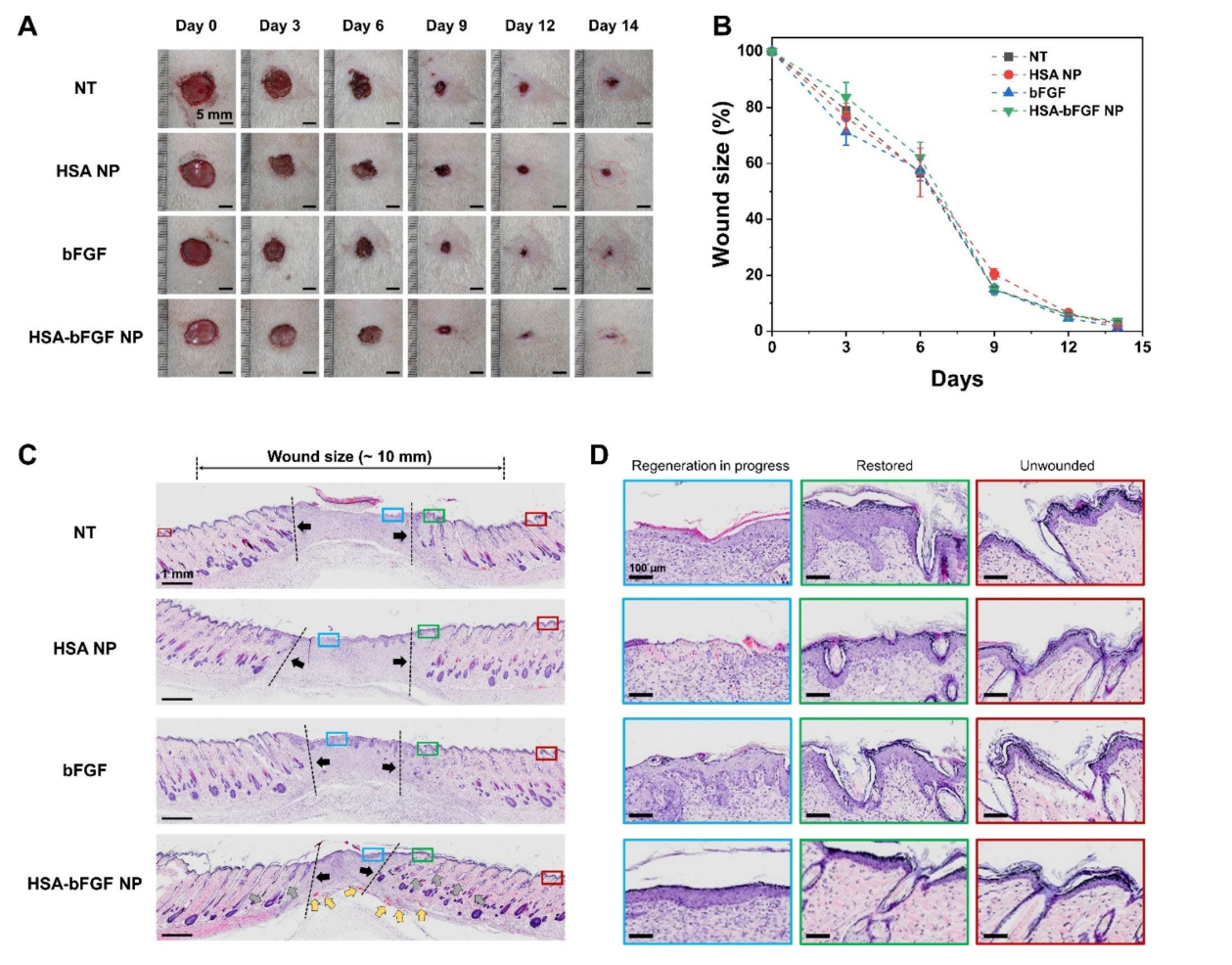
Figure 6. In vivo wound healing activity and histological evaluation of wound sections
Data Collection and Analysis
Data were collected at each stage using various methods. For in vitro assays, optical density measurements from CCK-8 and BrdU assays were analyzed using a microplate reader. Migration results from wound closure assays were quantified using ImageJ software. In vivo wound healing data were recorded through photographic documentation and analyzed using statistical software to determine significance across treatment groups. Histological evaluations were performed by counting the number of blood vessels and assessing collagen deposition through microscopic analysis.
Results
Key results showed that HSA-bFGF NPs significantly enhanced fibroblast proliferation and migration compared to controls. Specifically, treatment with HSA-bFGF NPs resulted in a greater than 50% increase in cell viability over five days. In vivo, HSA-bFGF NPs promoted superior wound closure rates and improved tissue regeneration, as evidenced by increased hair follicle and blood vessel formation, compared to soluble bFGF and control groups.
Novel Aspects
This study presents several novel aspects, including the successful production of soluble bFGF from a genetically engineered E. coli strain, which simplifies the production process and increases yield. The use of HSA as a stabilizing agent for bFGF in nanoparticle form provides a significant advantage over traditional delivery systems by offering controlled, sustained release of the growth factor, minimizing the risk of burst release and enhancing overall tissue regeneration efficacy. This innovative approach highlights the potential of HSA-bFGF NPs as a reliable delivery vehicle in regenerative medicine, paving the way for future applications in wound healing and tissue repair.
Conclusion
The successful development of the human serum albumin-based nanoparticles (HSA-bFGF NPs) as a drug delivery system for basic fibroblast growth factor (bFGF) has been achieved through a meticulous process involving the production of soluble bFGF, the formulation of stable nanoparticles, and extensive in vitro and in vivo evaluations. By employing a desolvation method to encapsulate bFGF within HSA nanoparticles, the research team was able to enhance the stability and sustained release profile of the growth factor, addressing the critical challenges associated with its short half-life and rapid degradation in physiological conditions.
The study highlights several significant findings, including the observation that HSA-bFGF NPs not only improved fibroblast proliferation and migration but also significantly reduced scar formation risk compared to traditional bFGF delivery methods. Furthermore, in vivo experiments demonstrated that these nanoparticles facilitated superior wound healing and tissue regeneration, evidenced by enhanced angiogenesis and improved tissue structure. Overall, the innovative use of HSA as a delivery vehicle for bFGF represents a promising advancement in regenerative medicine, providing a reliable and effective strategy for improving therapeutic outcomes in wound healing.
Reference
Son, Boram, et al. “Secured delivery of basic fibroblast growth factor using human serum albumin-based protein nanoparticles for enhanced wound healing and regeneration.” Journal of Nanobiotechnology, vol. 21, no. 310, 2023, https://doi.org/10.1186/s12951-023-02053-4.
