Editor: Nina
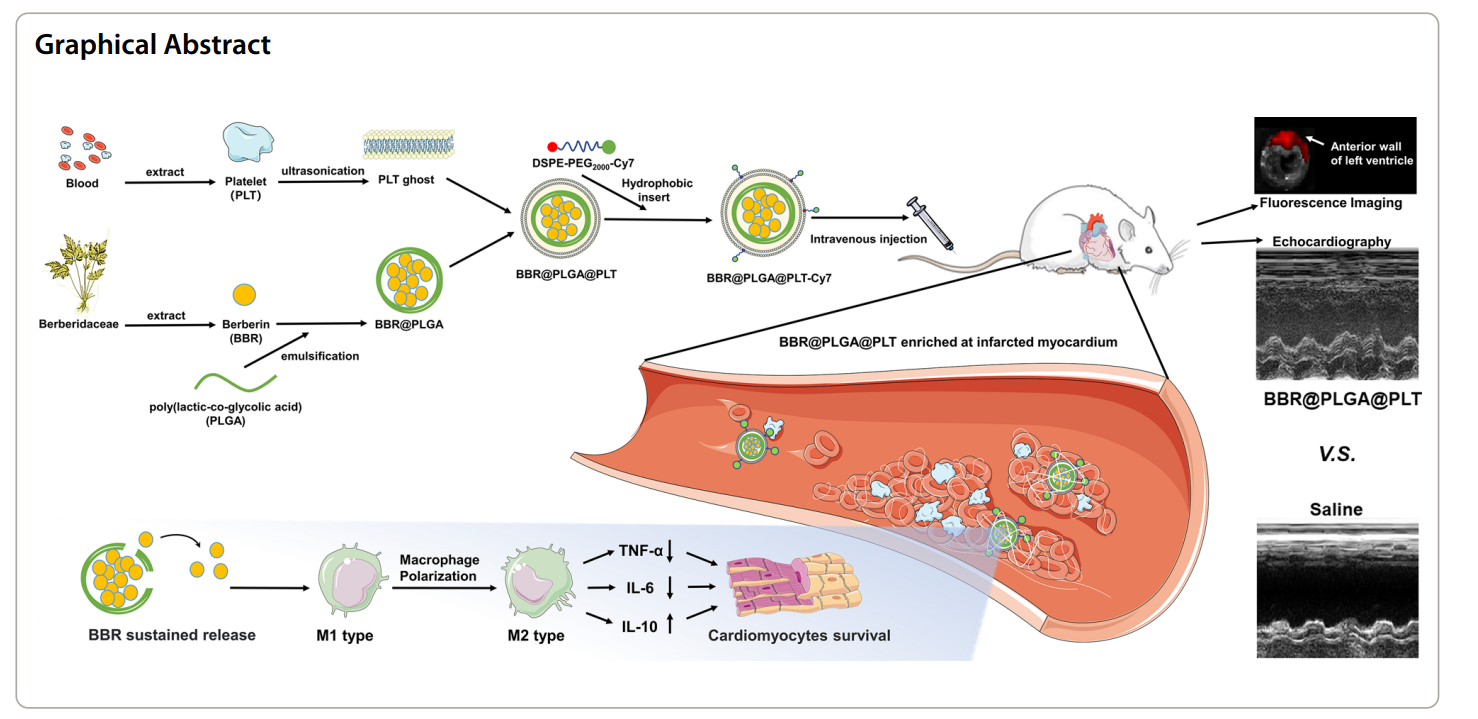
Scientists develop Berberin-loaded platelet membrane-coated PLGA nanoparticles for targeted and sustained drug delivery to enhance inflammation resolution and improve cardiac function following myocardial infarction.
Key Preview
- Research Question: This study investigates the potential of Berberin (BBR) loaded in platelet membrane-coated polylactic-co-glycolic acid (PLGA) nanoparticles (BBR@PLGA@PLT NPs) to enhance targeted delivery and sustained release of BBR in the infarcted myocardium, aiming to regulate inflammation and improve cardiac function after myocardial infarction (MI).
- Research Design and Strategy: The researchers utilized a combination of network pharmacology, in vitro assays, and in vivo experiments in a rat model of MI to assess the efficacy of BBR@PLGA@PLT NPs compared to conventional PLGA nanoparticles.
- Method: The study involved characterizing the nanoparticles, assessing their biodistribution, and evaluating their impact on inflammation resolution, cardiac function, and structural integrity through various biochemical and histological analyses over 28 days post-MI.
- Key Results: BBR@PLGA@PLT NPs demonstrated significantly enhanced accumulation in infarcted myocardium and reduced liver uptake compared to PLGA nanoparticles. Administration led to a marked increase in reparative macrophages and decreased inflammatory markers, alongside improved cardiac function and reduced collagen deposition in the heart.
- Significance of the Research: This research highlights a novel therapeutic strategy for MI that leverages biomimetic nanoparticles for targeted drug delivery, representing a significant advance in the field of cardiovascular therapeutics.
Introduction
Myocardial infarction (MI), commonly known as a heart attack, occurs when blood flow to a portion of the heart muscle is obstructed, leading to tissue damage and potential loss of cardiac function. This condition is a leading cause of morbidity and mortality worldwide, often resulting from atherosclerosis, thrombosis, or coronary artery disease. The aftermath of MI is characterized by an inflammatory response that, while essential for initial healing, can become excessive and detrimental if not properly regulated. This inflammation can exacerbate tissue damage, promote fibrosis, and lead to adverse remodeling of the heart, ultimately resulting in heart failure.
Traditional treatment strategies for MI typically involve pharmacological interventions aimed at restoring blood flow and managing symptoms. Commonly used drugs include antiplatelet agents, thrombolytics, beta-blockers, and ACE inhibitors. These medications are often administered systemically, which can provide some level of benefit but may also lead to suboptimal drug concentrations at the site of injury. Moreover, the non-specific distribution of these drugs can result in systemic side effects and reduced efficacy, as the therapeutic agents may not reach the infarcted myocardium in sufficient quantities.
The challenges associated with current drug delivery methods are significant. The low bioavailability of many cardiac drugs and their rapid clearance from the bloodstream can limit therapeutic effectiveness. Additionally, traditional intravenous administration can lead to adverse reactions, such as hypotension or organ toxicity, complicating treatment and further impacting patient outcomes. These issues highlight the need for innovative drug delivery systems that can enhance the targeting and retention of therapeutic agents specifically at the site of myocardial injury.
To address these challenges, researchers are exploring advanced drug delivery strategies that utilize biomimetic nanoparticles. One promising approach involves coating polylactic-co-glycolic acid (PLGA) nanoparticles with platelet membranes, creating a novel formulation known as Berberin-loaded PLGA nanoparticles coated with platelet membranes (BBR@PLGA@PLT NPs). This innovative strategy capitalizes on the natural ability of platelets to target sites of inflammation and injury, allowing for enhanced accumulation of the therapeutic agent in the infarcted myocardium. Not only does this method improve the bioavailability of Berberin, a natural compound with anti-inflammatory properties, but it also reduces systemic side effects associated with traditional delivery methods, paving the way for more effective treatments for myocardial infarction.
Research Team and Aim
The research team behind this study is composed of experts from Tongji Medical College, Huazhong University of Science and Technology, including Ke Zhu, Yu Yao, Kun Wang, and several other contributors. The lead researcher, Ke Zhu, conducted this research in collaboration with his colleagues in 2023. The findings of their study are documented in the paper titled “Berberin sustained-release nanoparticles were enriched in infarcted rat myocardium and resolved inflammation,” published in the Journal of Nanobiotechnology.
The primary aim of the research, as articulated by Ke Zhu, is to investigate the potential of Berberin-loaded polylactic-co-glycolic acid (PLGA) nanoparticles coated with platelet membranes (BBR@PLGA@PLT NPs) to enhance targeted drug delivery and sustained release of Berberin in the infarcted myocardium, thereby regulating inflammation and improving cardiac function following myocardial infarction (MI).
Experimental Process
Primary Technique:
The primary technique employed in this study is the formulation of Berberin-loaded polylactic-co-glycolic acid (PLGA) nanoparticles coated with platelet membranes (BBR@PLGA@PLT NPs). This technique was chosen to enhance the targeted delivery and sustained release of Berberin in the infarcted myocardium, thereby facilitating effective regulation of inflammation and improving cardiac function after myocardial infarction (MI).
Key Steps of Each Experiment:
- Nanoparticle Preparation:
- Step 1: 500 mg of PLGA (lactide/glycolide ratio of 75:25, Mw: 4k–15k Da) was dissolved in 10 mL of dichloromethane.
- Step 2: A freshly prepared Berberin (BBR) solution (containing 100 mg BBR, 300 µL DMSO, and 4.7 mL ultrapure water) was added to the PLGA solution.
- Step 3: The mixture was sonicated in an ice bath for 500 seconds using a probe sonicator (10% amplitude).
- Step 4: The resulting W1/O emulsion was rapidly incorporated into 25 mL of 1% PVA1788 solution and homogenized at high speed (12000 rpm) for 10 minutes in an ice bath.
- Step 5: The suspension was then added to 30 mL of 3% isopropanol and stirred overnight to evaporate the dichloromethane.
- Step 6: The nanoparticles were washed three times by centrifugation (12000 g for 20 minutes) and lyophilized for storage.
Results
BBR@PLGA@PLT NPs exhibited a hydrodynamic diameter of approximately 235 nm and a controlled release profile, with only 34.90% of BBR released after 10 days compared to 79.83% from non-coated PLGA nanoparticles.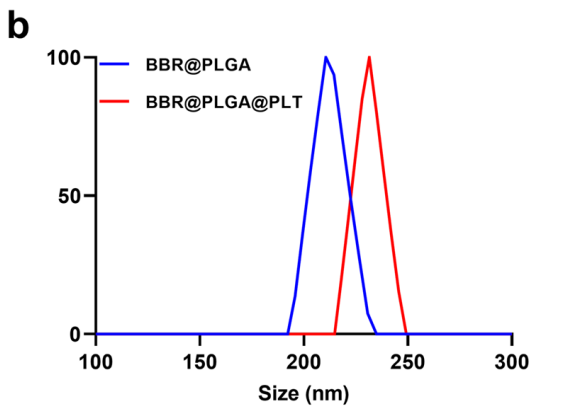
Figure 1.The sizes of BBR@PLGA and BBR@PLGA@PLT NPs.
- Characterization of Nanoparticles:
- Step 1: Transmission electron microscopy (TEM) was employed to examine the morphology of BBR@PLGA and BBR@PLGA@PLT NPs.
- Step 2: Dynamic light scattering (DLS) was utilized to assess the size, polydispersity index, and zeta potential of both nanoparticle types.
- Step 3: Stability tests were conducted over an eight-day period to monitor changes in size and zeta potential.
- Step 4: In vitro drug release kinetics were evaluated by incubating the nanoparticles in phosphate-buffered saline (PBS) and measuring the cumulative release of Berberin over 20 days.
Results
TEM images confirmed the successful coating of nanoparticles, while DLS analysis demonstrated favorable stability with no significant size or zeta potential changes over eight days.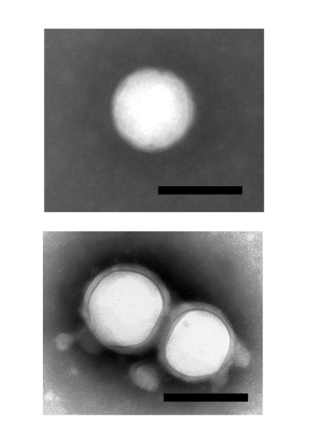
Figure 2. Transmission electron microscopy images of BBR@PLGA and BBR@PLGA@PLT NPs (scale bar = 200 nm).
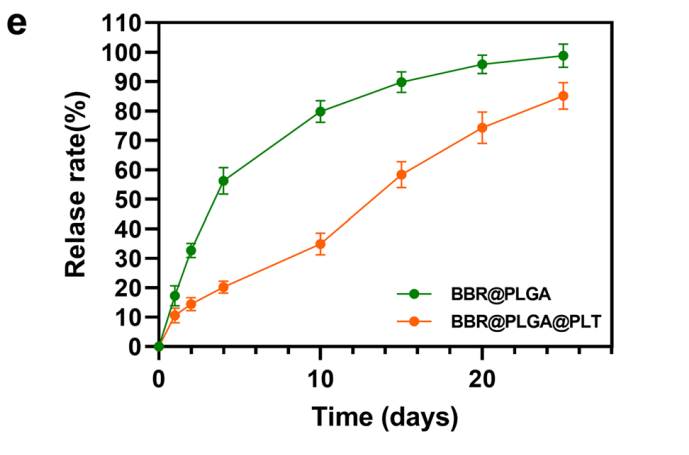
Figure 3. In vitro drug release profles of BBR@PLGA and BBR@PLGA@PLT NPs.
- Biodistribution Studies:
- Step 1: BBR@PLGA@PLT NPs were labeled with DSPE-PEG2000-Cy7 for fluorescence imaging.
- Step 2: Following intravenous administration into MI rat models, major organs (heart, liver, kidney, spleen, and lungs) were harvested 24 hours post-injection.
- Step 3: Ex vivo fluorescence imaging was performed using the IVIS® Spectrum In Vivo Imaging System to determine the distribution of nanoparticles.
Results
Fluorescence imaging indicated significantly enhanced accumulation of BBR@PLGA@PLT NPs in the infarcted myocardium, with reduced liver uptake compared to uncoated PLGA nanoparticles.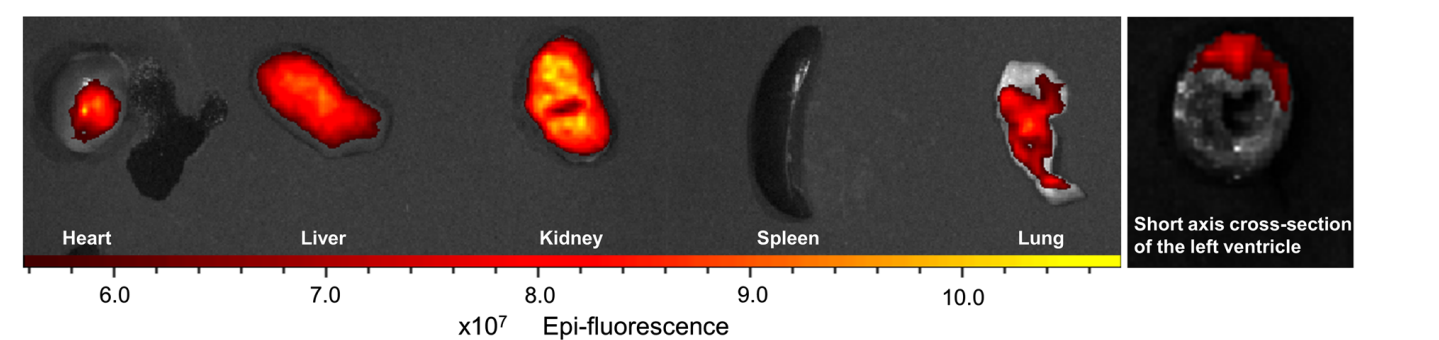
Figure 4. Ex vivo fuorescence imaging of the heart and the other major organs (liver, kidney, spleen, and lung) of MI rats 24 h after DSPE-PEG2000-Cy7-labelled BBR@PLGA@PLT administration. All bars represent as means ± SD (n = 3)
- In Vivo Efficacy Assessment:
- Step 1: MI was induced in male Sprague Dawley rats by ligating the left anterior descending coronary artery.
- Step 2: Post-surgery, rats were treated with BBR@PLGA@PLT NPs (containing 0.8 mg of BBR) or control formulations via tail vein injection.
- Step 3: Cardiac function was evaluated via echocardiography on day 28 after MI, measuring parameters such as left ventricular ejection fraction (LVEF) and left ventricular internal diameter (LVID).
- Step 4: Histological analysis of myocardial tissue was conducted to assess collagen deposition and inflammatory response.
Results
Echocardiographic analysis revealed that the BBR@PLGA@PLT treatment led to a marked improvement in LVEF and a reduction in ventricular dimensions, alongside decreased collagen deposition in myocardial tissue.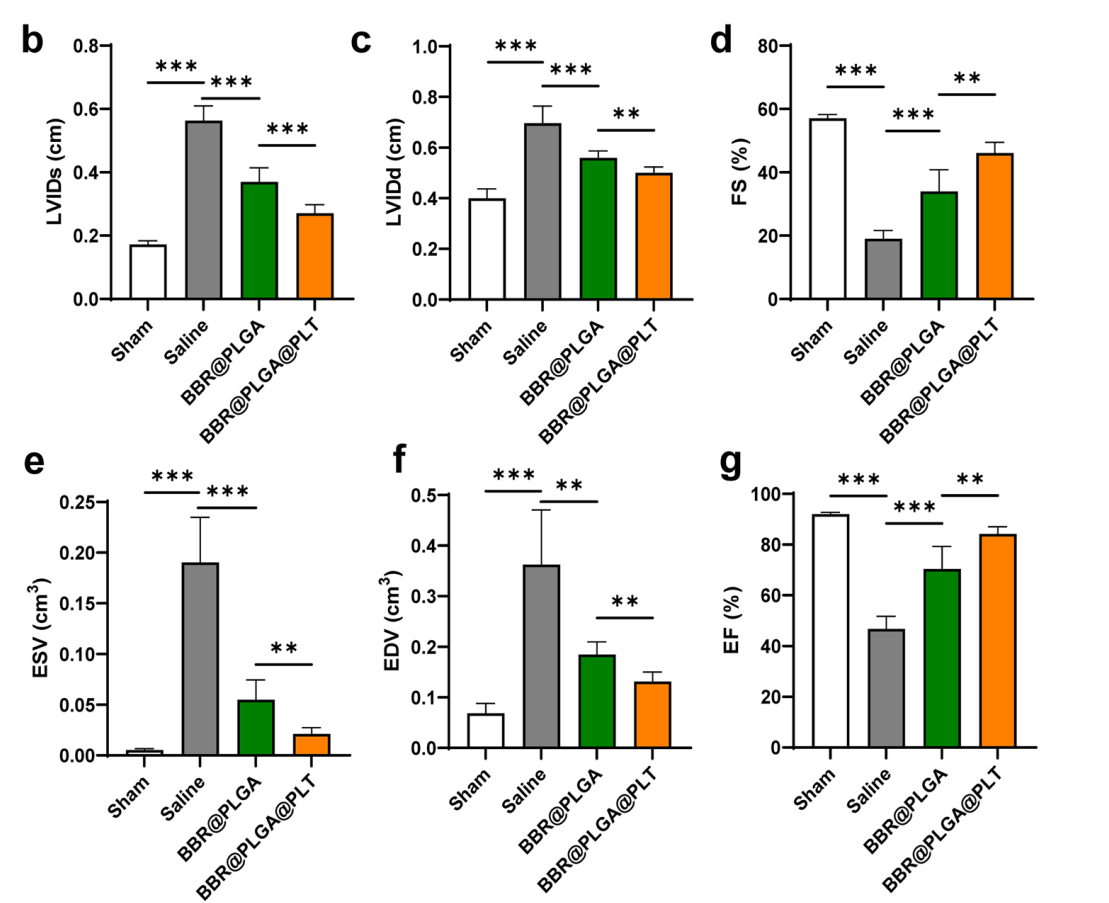
Figure 5. Cardiac function was evaluated by echocardiography on day 28 after MI, b LVIDs. c LVIDd. d FS. e ESV. f EDV. g LVEF. All bars represent as means ± SD (n = 6). *P < 0.05 and **P < 0.01, ***P < 0.001
Data Collection and Analysis:
Data were collected through a combination of fluorescence imaging, echocardiographic assessments, and biochemical assays. For in vivo studies, fluorescence intensity was quantified using the IVIS Imaging System. Echocardiographic parameters were analyzed using the Philips EPIQ5 system, and statistical analysis was performed using one-way ANOVA with a significance threshold set at p < 0.05. Histological samples were quantified using ImageJ software to evaluate collagen content and myocardial architecture.
Novel Aspects and Advantages:
The study introduced a novel therapeutic strategy by utilizing platelet membrane-coated nanoparticles for targeted drug delivery. This approach enhances the bioavailability of Berberin while minimizing side effects associated with intravenous administration. The biomimetic nature of the platelet coating promotes accumulation in inflammatory lesions, improving the therapeutic efficacy of the drug compared to traditional nano-delivery systems, which often lack such targeted capabilities. This study demonstrates a significant advancement in the development of nanomedicines for cardiac applications, highlighting the potential for improved patient outcomes in myocardial infarction treatment.
Conclusion
The successful development of the Berberin-loaded polylactic-co-glycolic acid (PLGA) nanoparticles coated with platelet membranes (BBR@PLGA@PLT NPs) as a drug delivery system is achieved through a combination of innovative formulation techniques and a thorough understanding of the inflammatory processes involved in myocardial infarction (MI). By leveraging the natural targeting ability of platelets, the researchers were able to create a biomimetic nanoparticle system that enhances the accumulation of Berberin specifically in the infarcted myocardium. This strategic approach not only improves the bioavailability of the therapeutic agent but also facilitates its sustained release, providing a more effective treatment option for managing post-MI inflammation and cardiac repair.
The highlights of the study include the demonstration that BBR@PLGA@PLT NPs significantly enhance drug accumulation in the infarcted myocardium while reducing liver uptake compared to conventional PLGA nanoparticles. The administration of these nanoparticles resulted in a marked increase in reparative macrophages, a decrease in inflammatory markers, and notable improvements in cardiac function and tissue integrity. Overall, this research presents a promising advancement in the field of cardiovascular therapeutics, offering a novel solution for the treatment of myocardial infarction that could lead to improved patient outcomes.
Reference
Zhu, Ke, et al. “Berberin Sustained-Release Nanoparticles Were Enriched in Infarcted Rat Myocardium and Resolved Inflammation.” Journal of Nanobiotechnology, vol. 21, no. 33, 2023, https://doi.org/10.1186/s12951-023-01790-w.
