Editor: Tiffany
A new study introduces an intranasal nanogel delivery system for propranolol, improving its brain availability and presenting a promising option for enhancing migraine treatment efficacy.
Key Highlights
- Research Question: How can propranolol delivery be improved to address its limitations in oral administration for migraine treatment?
- Research Difficulties: Challenges include low oral bioavailability, extensive hepatic first-pass metabolism, and the need for frequent dosing with propranolol.
- Key Findings: The intranasal nanogel formulation of propranolol showed enhanced nasal permeation, safety, and significantly increased brain delivery compared to its oral form.
- Innovative Aspects: The study employs a limonene-based microemulsion integrated into a thermo-responsive mucoadhesive nanogel for intranasal administration, offering a novel delivery method.
- Importance of the Study: This approach provides a potential solution to current migraine treatment limitations, improving effectiveness and patient convenience.
Migraine Challenges and Propranolol Limitations
Migraine is a widespread neurological disorder affecting millions globally, characterized by intense, often unilateral headaches that can persist from hours to days. According to a study published in Gels in 2023 by Kawthar K. Abla, Souraya Domiati, Rania El Majzoub, and Mohammed M. Mehanna, migraines are frequently accompanied by symptoms such as nausea, vomiting, and heightened sensitivity to light and sound, with some patients experiencing visual disturbances known as aura. These debilitating symptoms significantly impair quality of life, necessitating effective therapeutic strategies.
Current migraine management includes acute treatments such as triptans, non-steroidal anti-inflammatory drugs (NSAIDs), and anti-emetics to relieve symptoms during an attack. For prophylaxis, beta-blockers like propranolol are widely regarded as effective. However, the study highlights that propranolol’s oral administration is hindered by low bioavailability (15-23%) due to extensive hepatic first-pass metabolism, requiring frequent dosing that may reduce patient adherence and lead to inconsistent therapeutic outcomes.
Designing an Intranasal Propranolol Delivery System
To address these challenges, the research team from Beirut Arab University, Lebanese International University, and Alexandria University developed a novel intranasal delivery system for propranolol. The primary objective, as outlined in their 2023 Gels publication, was to enhance brain delivery of propranolol, bypassing hepatic metabolism, to improve efficacy and minimize systemic side effects. This approach leverages the nasal route’s direct access to the central nervous system, aiming to provide a more efficient and patient-friendly alternative for migraine prevention.
Nanogel Formulation and Performance Outcomes
The study employed a multi-faceted approach to design, characterize, and evaluate a propranolol-loaded limonene-based microemulsion integrated into a thermo-responsive mucoadhesive nanogel. Below are the detailed methods and findings from each experimental phase, as reported in the paper.
1. Microemulsion Characterization
Procedure: The microemulsion was formulated using limonene and Gelucire 44/14 as the oily phase, Labrasol and Labrafil as surfactants, and deionized water as the aqueous phase, with propranolol dissolved in the water component.
Result: The resulting microemulsion had a nanometric droplet size of 133.7 nm, a polydispersity index (PDI) of 0.112, a zeta potential of -7.2 mV, an entrapment efficiency of 81.9%, and a drug loading capacity of 117 mg/g.
Finding: These properties indicate a stable, uniform nanosystem with high drug incorporation, suitable for effective nasal delivery.
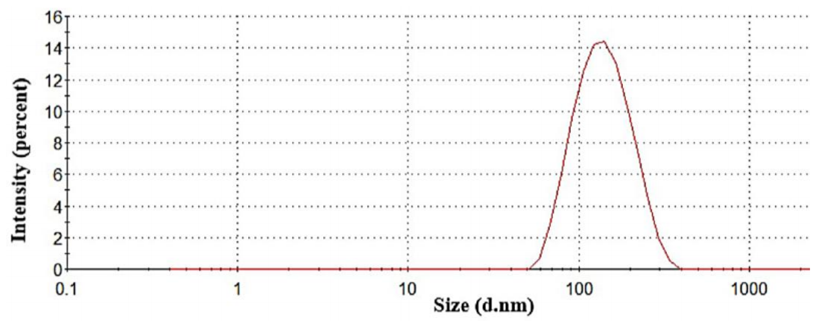
Figure 1. Particle size distribution of propranolol-loaded limonene-based microemulsion.
2. Nanogel Properties
Procedure: The microemulsion was incorporated into a nanogel using poloxamer (26% w/w) and chitosan (1% w/w), optimized for thermo-responsive and mucoadhesive characteristics.
Result: The nanogel exhibited gelation at 30-34°C, a rapid gelation time, a pH of 6.08, increased viscosity upon gelation, and strong mucoadhesion to nasal mucosa.
Finding: These attributes ensure the formulation remains liquid at room temperature for ease of administration and transitions to a gel at nasal temperature, prolonging drug residence time and enhancing absorption.
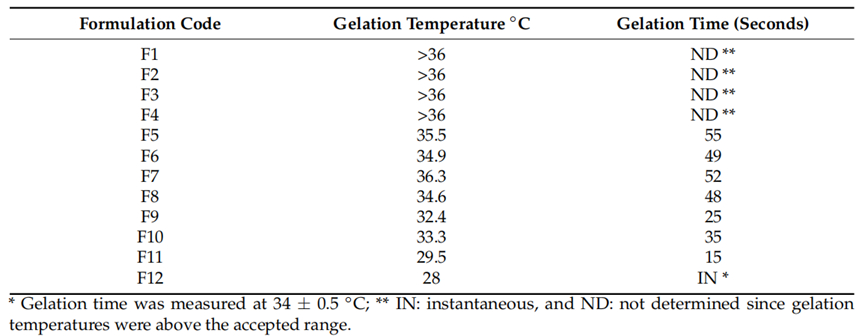
Table 1. Gelation temperature and gelation time* of the prepared formulations.
3. In Vitro Release
Procedure: Drug release was assessed using dialysis bags in phosphate buffer at 34°C, mimicking nasal conditions.
Result: The nanogel demonstrated controlled release of propranolol over 4 hours, with a mean release time of 1.48 hours, compared to 1.21 hours for a control gel.
Finding: This sustained release profile suggests the nanogel can maintain therapeutic drug levels over an extended period, potentially reducing dosing frequency.
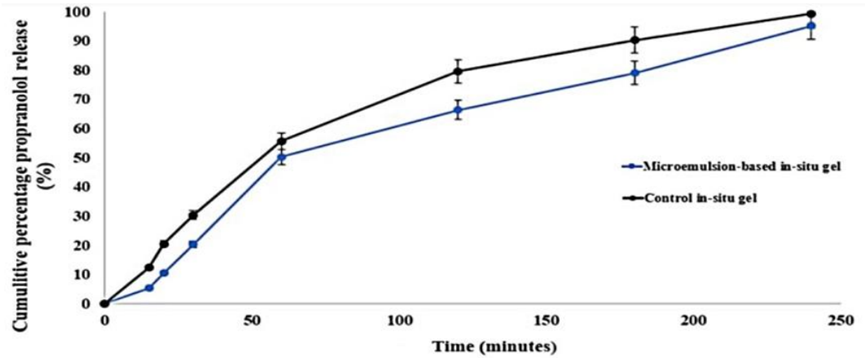
Figure 2. The in vitro release pattern.
4. Ex Vivo Permeation
Procedure: Permeation studies utilized sheep nasal mucosa in Franz diffusion cells to evaluate drug transport.
Result: The nanogel achieved a cumulative permeation of 603.55 µg/cm² and a flux of 1.56 µg/cm²·min, significantly higher than the control gel’s 420.3 µg/cm² and 1.24 µg/cm²·min, respectively.
Finding: The enhanced permeation underscores the microemulsion-based nanogel’s ability to facilitate propranolol transport across nasal tissue, a critical factor for effective brain delivery.
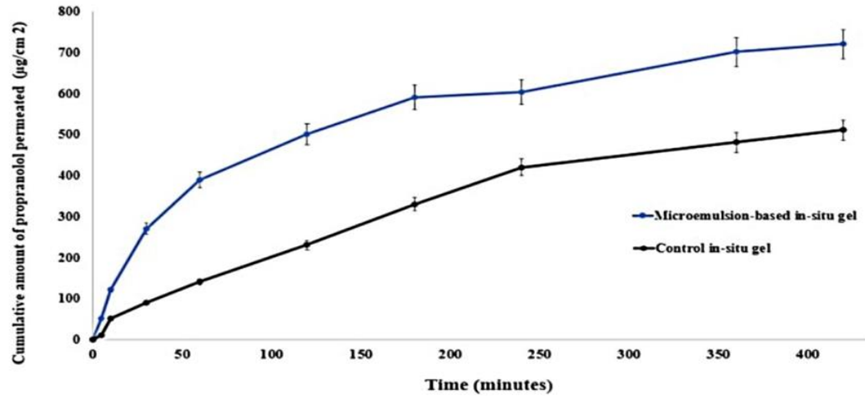
Figure 3. The ex vivo permeation behavior.
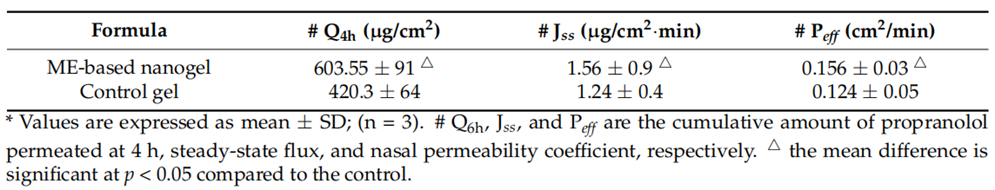
Table 2. Nasal permeation parameters of propranolol-loaded limonene-based microemulsion thermos-responsive nanogel and the control gel.
5. Brain Uptake Study
Procedure: In vivo studies in rats compared brain concentrations following intranasal nanogel administration to those from an oral propranolol solution.
Result: The intranasal nanogel yielded a maximum brain concentration (Cmax) of 970.3 ng/g and an area under the curve (AUC) of 4884.15 ng·h/g, markedly higher than the oral solution’s 277.7 ng/g and 1518.35 ng·h/g, respectively.
Finding: These results confirm the intranasal system’s superior brain-targeting efficiency, highlighting its potential to enhance propranolol’s therapeutic impact in migraine management.
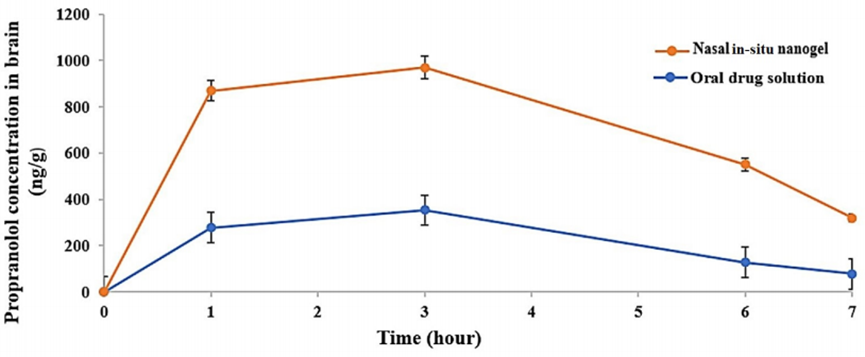
Figure 4. Propranolol behavior in the brain with respect to time after optimized limonene-based microemulsion in situ gel and oral drug solution at a dose of 20 mg/kg (mean values ± SD (n = 4)).
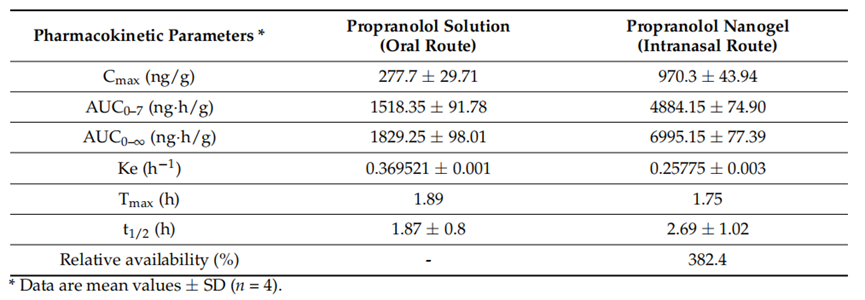
Table 3. Pharmacokinetic parameters of propranolol in the brain after oral solution and intranasal microemulsion-based in situ nanogel administration.
Impact on Migraine Therapy and Next Steps
The research, published in Gels in 2023, successfully developed an innovative intranasal delivery system for propranolol, combining a limonene-based microemulsion with a thermo-responsive mucoadhesive nanogel. This formulation exhibited optimal physicochemical properties, including nanoscale size, high drug entrapment, and stability, alongside favorable nasal administration characteristics such as temperature-dependent gelation and strong mucoadhesion. In vitro studies demonstrated controlled drug release over 4 hours, while ex vivo experiments confirmed enhanced permeation through nasal mucosa. Most notably, in vivo rat studies revealed significantly higher brain concentrations and bioavailability compared to oral administration.
This novel system addresses key limitations of oral propranolol, such as low bioavailability and frequent dosing, by leveraging the nasal route’s direct access to the brain. The enhanced brain delivery, coupled with sustained release and improved permeation, positions this formulation as a promising advancement in migraine prophylaxis. The researchers conclude that the propranolol-loaded nanogel offers a safe, effective, and patient-friendly alternative, potentially revolutionizing the management of this debilitating neurological condition.
Reference:
Abla, Kawthar K., et al. “Propranolol-loaded limonene-based microemulsion thermo-responsive mucoadhesive nasal nanogel: design, in vitro assessment, ex vivo permeation, and brain biodistribution.” Gels 9.6 (2023): 491.
