Editor: Tiffany
Researchers have developed an innovative combination of lactoferrin-coated selenium nanoparticles that demonstrates significant anticancer activity, selectively targeting various human cancer cells while sparing healthy tissues.
Key Highlights
- Research Question:
Can lactoferrin-coated selenium nanoparticles enhance the selectivity and efficacy of anticancer treatments against invasive cancer cells? - Research Difficulties:
Achieving high selectivity for cancer cells while minimizing toxicity to normal cells has been a significant challenge in developing effective cancer therapies. - Key Findings:
The lactoferrin-coated selenium nanoparticles (ALF-Se NPs) exhibited a remarkable anti-proliferation efficiency against human cancer cell lines, including MCF-7, HepG-2, and Caco-2, with an IC50 of less than 64 μg/mL, significantly outperforming free lactoferrin and selenium nanoparticles alone. - Innovative Aspects:
This study introduces a novel method of bio-synthesizing selenium nanoparticles using Rhodotorula sp. and demonstrates their enhanced anticancer properties when combined with bovine lactoferrin. - Importance of the Study:
These findings could pave the way for developing more selective and effective cancer therapies, potentially improving patient outcomes and reducing side effects associated with traditional chemotherapy.
Cancer Treatment Challenges and the Role of Lactoferrin
Cancer remains a significant global health issue, with breast, liver, and colon cancers ranking among the most common and lethal types. In 2020, breast cancer affected approximately 2.2 million individuals worldwide, while liver cancer and colon cancer accounted for 0.905 million and 1.15 million cases, respectively. These cancers present diverse symptoms, such as fatigue, unintended weight loss, and localized pain, which vary depending on the cancer type and its progression. A major challenge in treating these diseases is their ability to metastasize, spreading from the primary site to other organs, which complicates therapeutic strategies.
Chemotherapy is a cornerstone of cancer treatment, functioning by inhibiting the growth and division of cancer cells. Despite its widespread use, it has notable drawbacks, including systemic side effects, the development of drug resistance, high toxicity, and limited selectivity. These limitations often result in damage to healthy cells, reducing patient quality of life and treatment efficacy. Consequently, there is a pressing need for new approaches that can target cancer cells more effectively while sparing healthy tissues.
Creation of Lactoferrin-Coated Selenium Nanoparticles
A team of scientists from institutions in Egypt and Saudi Arabia, including Esmail M. El-Fakharany and colleagues, set out to address these challenges by developing a new treatment option. Their study, published in Scientific Reports in 2023, focused on creating a nanocombination that combines bovine lactoferrin (bLF)—a protein found in cow’s milk—with selenium nanoparticles (Se NPs). The primary aim was to design a treatment that could selectively attack cancer cells and trigger apoptosis, a process where cells are programmed to die, thus halting cancer progression.
The researchers had several specific objectives. They planned to biosynthesize selenium nanoparticles, purify bLF from milk to create an iron-free form called apo-lactoferrin (ALF), and conjugate these components into a stable nanocombination, termed ALF-Se NPs. Their goal was to test this nanocombination’s effectiveness against breast, liver, and colon cancer cells, comparing its performance to the individual components (Se NPs and ALF alone). By doing so, they hoped to demonstrate a more potent and selective approach to cancer therapy.
Characterization and Efficacy of ALF-Se NPs
The research involved a series of carefully designed steps and experiments to create and evaluate the ALF-Se NPs. Below are detailed descriptions of the key experiments, including their procedures, results, and new findings.
1. Synthesis and Characterization of Selenium Nanoparticles (Se NPs)
- Procedure: The team used a yeast strain, Rhodotorula sp., to biosynthesize Se NPs. They mixed selenium salts with the yeast in a controlled environment, allowing the yeast to reduce the salts into nanoparticles. These particles were then purified and analyzed using techniques like scanning electron microscopy (SEM) and transmission electron microscopy (TEM) to determine their size, shape, and composition.
- Result: The analysis showed that the Se NPs were uniform spheres ranging from 18 to 40 nanometers in size, with consistent structural properties.
- New Finding: This experiment confirmed that yeast could effectively produce small, stable Se NPs suitable for medical applications, providing a reliable foundation for the nanocombination.
2. Conjugation of Se NPs with Apo-Lactoferrin (ALF-Se NPs)
- Procedure: The researchers first extracted bLF from defatted cow’s milk using cation exchange chromatography, ensuring it was free of iron (apo-lactoferrin, or ALF). They then embedded the biosynthesized Se NPs into ALF using a specific conjugation method. The resulting ALF-Se NPs were characterized with SEM, TEM, and Fourier-transform infrared spectroscopy (FTIR) to verify the integration and structure.
- Result: The characterization revealed spherical ALF-Se NPs with sizes less than 200 nanometers, indicating successful conjugation of Se NPs with ALF while maintaining structural integrity.
- New Finding: The study demonstrated that Se NPs could be stably integrated into ALF, creating a nanocombination with distinct physical properties compared to its individual components, setting the stage for functional testing.
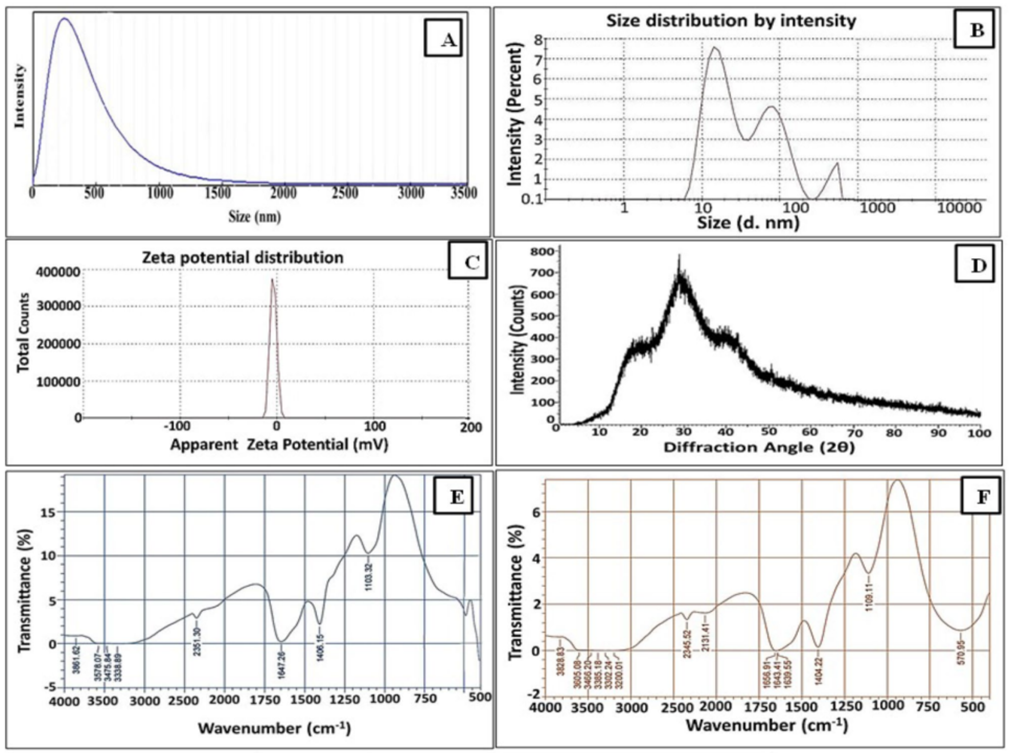
Figure 1. Compositional, structural and functional features of Se NPs synthesized by Rhodotorula sp. MNR.
3. Cytotoxicity Testing on Cancer Cell Lines
- Procedure: The team tested ALF-Se NPs on three cancer cell lines—breast (MCF-7), liver (HepG-2), and colon (Caco-2)—using the MTT assay, which measures cell viability. They treated the cells with varying concentrations of ALF-Se NPs, Se NPs alone, and ALF alone, then calculated the IC50 value (the concentration needed to inhibit 50% of cell growth) for each.
- Result: ALF-Se NPs showed a stronger anticancer effect, with IC50 values below 64 micrograms per milliliter across all cell lines, compared to higher values for Se NPs and ALF alone. This indicated greater potency.
- New Finding: The nanocombination outperformed its individual components, suggesting a synergistic effect between ALF and Se NPs that enhances their ability to kill cancer cells.
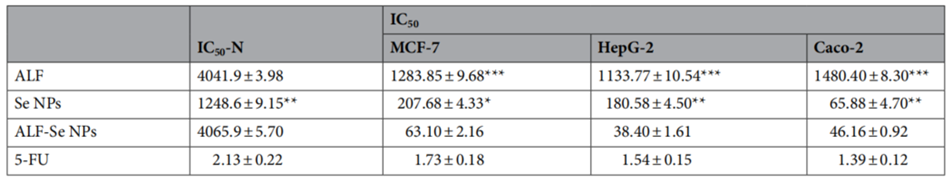
Table 1. Cytotoxicity of ALF with Se NPs in the term of IC50-N (μg/ml) and IC50 (μg/ml) against human normal cells (Wi-38) and cancer cells as compared to 5-FU.
4. Apoptosis Assays to Assess Cell Death
- Procedure: To explore how ALF-Se NPs kill cancer cells, the researchers used flow cytometry and fluorescence microscopy. They treated the cancer cells with ALF-Se NPs and stained them to detect signs of apoptosis, such as DNA fragmentation and cell membrane changes, then quantified the percentage of apoptotic cells.
- Result: Approximately 48-53% of cells treated with ALF-Se NPs underwent apoptosis, compared to less than 10% with ALF alone and about 28% with Se NPs alone.
- New Finding: The nanocombination significantly increased apoptosis rates, providing evidence that it actively triggers programmed cell death in cancer cells, a key mechanism for halting tumor growth.
Additional tests examined molecular changes. Using gene expression analysis, the team found that ALF-Se NPs increased levels of p53—a gene that suppresses tumors—by over eightfold in colon cancer cells, while reducing levels of Bcl-2, MMP-9, and VEGF, which support cancer survival and spread. The treatment also lowered reactive oxygen species (ROS) levels and activated Nrf2, a factor involved in managing oxidative stress, further contributing to its anticancer effects.
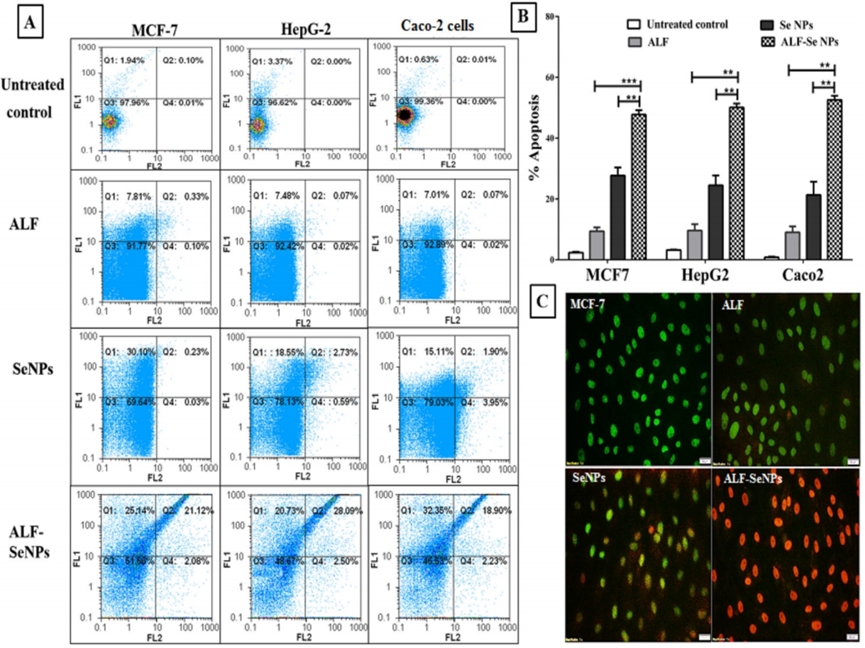
Figure 2. Apoptotic efect of ALF-Se NPs in comparison with ALF and Se NPs.
Conclusion: ALF-Se NPs as a Targeted Cancer Therapy
This research successfully developed and tested a nanocombination of bovine lactoferrin and selenium nanoparticles (ALF-Se NPs) as a potential cancer treatment. The study showed that ALF-Se NPs effectively targeted breast, liver, and colon cancer cells, inducing apoptosis and modulating key cancer-related genes like p53 and Bcl-2, while showing minimal toxicity to healthy cells. The integration of bLF, known for its anticancer and immunomodulatory effects, with Se NPs, which provide high bioavailability, resulted in improved efficacy over the individual components.
The findings suggest that ALF-Se NPs could offer a more selective and effective alternative to conventional treatments like chemotherapy. The use of nanotechnology in this context enhances drug delivery and therapeutic outcomes, addressing some of the limitations of current cancer therapies. While promising, the study calls for further pre-clinical and clinical investigations to validate the safety and efficacy of ALF-Se NPs in vivo, potentially advancing their application in oncology.
Reference:
El Fakharany, Esmail M., et al. “Anticancer activity of lactoferrin-coated biosynthesized selenium nanoparticles for combating different human cancer cells via mediating apoptotic effects.” Scientific Reports 13.1 (2023): 9579.
