Scientists develop a dual-drug-loaded smart niosome-chitosan nanocarrier system for lung cancer treatment, offering enhanced drug delivery, pH and temperature responsiveness, and antibacterial activity.
Key Preview
- Research Question: How can a dual-drug-loaded delivery system enhance the treatment efficacy for lung cancer while simultaneously exhibiting antibacterial properties?
- Research Design and Strategy: The study employed a novel niosome-based delivery platform that encapsulates curcumin, an anticancer agent, and Rose Bengal, a photosensitizer with antibacterial properties, within a chitosan polymeric shell.
- Method: The researchers utilized the thin-film hydration method to fabricate curcumin-loaded niosomes, followed by the coating with a chitosan polymer. Various physicochemical characterizations were conducted, including size analysis, drug release studies, and cytotoxicity assessments against lung cancer cells and bacteria.
- Key Results: The dual-drug-loaded niosomes exhibited an average size of approximately 80 nm and high encapsulation efficiencies (97% for curcumin and 98% for Rose Bengal). The release profiles indicated pH and temperature responsiveness, with nearly 60% of Rose Bengal and 35% of curcumin released at 37 °C and pH 5.5. The formulation demonstrated about 50% toxicity against lung cancer cells over 72 hours.
- Significance of the Research: This innovative platform could lead to more efficient and targeted treatment approaches for lung cancer, addressing the challenges of low bioavailability and the need for antibacterial properties in cancer therapies.
Introduction
Lung cancer is one of the most prevalent and lethal forms of cancer globally, ranking as the leading cause of cancer-related deaths. The disease is primarily categorized into two main types: non-small-cell lung carcinoma (NSCLC), which accounts for approximately 80% of cases, and small-cell lung carcinoma (SCLC), which constitutes the remaining 20%. Factors such as smoking, environmental pollution, and genetic predispositions significantly contribute to the rising incidence of lung cancer. Unfortunately, the five-year survival rate remains alarmingly low, often attributed to late-stage diagnosis and the ineffectiveness of existing treatment modalities.
Traditional strategies for lung cancer treatment typically involve a combination of chemotherapy, radiation therapy, and, in some cases, targeted therapies. Chemotherapy is particularly favored for its ability to prevent cancer recurrence and metastasis. However, the delivery of chemotherapeutic agents is often hampered by their low bioavailability and limited solubility in aqueous environments. Moreover, these agents frequently exhibit systemic toxicity, affecting healthy cells and leading to significant side effects, which can severely diminish patients’ quality of life.
Current therapeutic approaches face several distinct challenges, including the rapid degradation of drugs in the bloodstream, insufficient targeting of cancerous tissues, and the emergence of drug resistance. These challenges hinder the effectiveness of treatment regimens, often resulting in suboptimal therapeutic outcomes and the progression of the disease. Additionally, the presence of bacterial infections in tumor tissues can complicate treatment, further exacerbating the need for innovative solutions.
To address these challenges, the present study introduces a novel drug delivery strategy based on a dual-drug-loaded niosome-g-chitosan polymeric platform. This innovative approach encapsulates curcumin, an anticancer agent, and Rose Bengal, a photosensitizer with antibacterial properties, within a chitosan-coated niosome. The use of niosomes enhances the solubility and stability of hydrophobic drugs, while the chitosan coating provides mucoadhesive properties and targeted delivery capabilities, particularly in the acidic microenvironment of cancer tissues. By combining these elements, the proposed system aims to improve therapeutic efficacy, minimize side effects, and combat bacterial infections, ultimately advancing the treatment landscape for lung cancer.
Research Team and Aim
The research team behind this innovative study is composed of a diverse group of experts from various institutions in Turkey. The lead researcher, Atefeh Zarepour, along with co-authors Abdurrahim Can Egil, Melike Cokol Cakmak, Monireh Esmaeili Rad, Yuksel Cetin, Seyma Aydinlik, Gozde Ozaydin Ince, and Ali Zarrabi, conducted this research at Istinye University and Sabanci University. The study, titled “Fabrication of a Dual-Drug-Loaded Smart Niosome-g-Chitosan Polymeric Platform for Lung Cancer Treatment,” was published in January 2023 in the journal Polymers.
The primary aim of the research, as articulated by the lead researcher, was to “develop a smart drug delivery platform that addresses the limitations of current lung cancer treatments, particularly focusing on enhancing efficacy and minimizing side effects.” This innovative approach aims to improve the bioavailability of therapeutic agents while simultaneously providing antibacterial properties, thereby offering a comprehensive solution to the multifaceted challenges in lung cancer treatment.
Experimental Process
Primary Technique
The primary technique employed in this research was the fabrication of a dual-drug-loaded niosome-g-chitosan polymeric platform. This innovative method combined the use of niosomes, which are nonionic surfactant-based vesicles, with a chitosan polymer coating to enhance the delivery of curcumin and Rose Bengal. The dual loading of these compounds aimed to achieve both anticancer and antibacterial properties, marking a significant advancement in drug delivery systems for lung cancer treatment.
Key Steps of Each Experiment
- Preparation of Curcumin-Loaded Niosomes:
- Span 60, cholesterol, and curcumin were dissolved in a chloroform-methanol mixture.
- The solution was evaporated to form a thin film using a rotary vacuum evaporator.
- Phosphate-buffered saline (PBS) was added to the thin film, followed by sonication at 60 °C for one hour to create curcumin-loaded niosomes.
- The solution was centrifuged at 12,000 RPM for 30 minutes to remove unencapsulated curcumin.
- Chitosan Coating:
- Chitosan was grafted with N-vinyl-caprolactam to create a polymer solution.
- This solution was mixed with the curcumin-loaded niosomes using a microfluidic syringe pump, allowing precise control over the coating process.
- The mixture was stirred for one hour and then centrifuged to remove excess polymer and unencapsulated Rose Bengal, resulting in chitosan-coated niosomes.
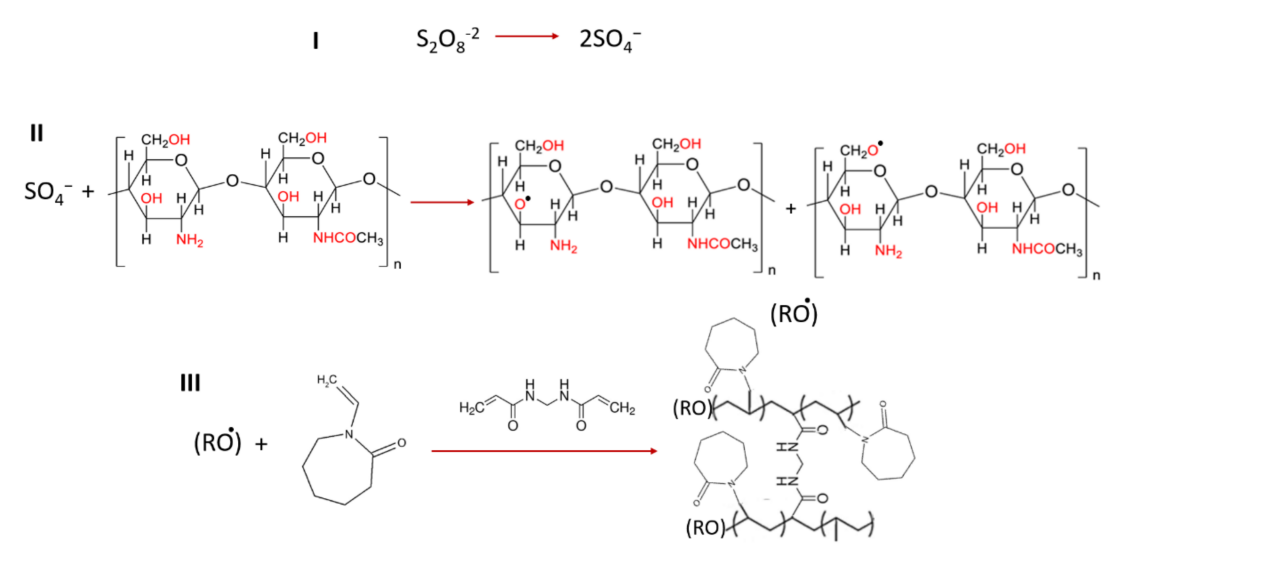
Scheme 1.Chemical reactions related to the preparation of grafted polymer.
- Characterization of Nanoparticles:
- Dynamic light scattering (DLS) was utilized to measure the hydrodynamic size, surface charge, and polydispersity index (PDI) of the nanoparticles.
- Field emission scanning electron microscopy (FE-SEM) and transmission electron microscopy (TEM) were employed to confirm the size and morphology of the niosomes, ensuring uniformity and stability of the formulation.
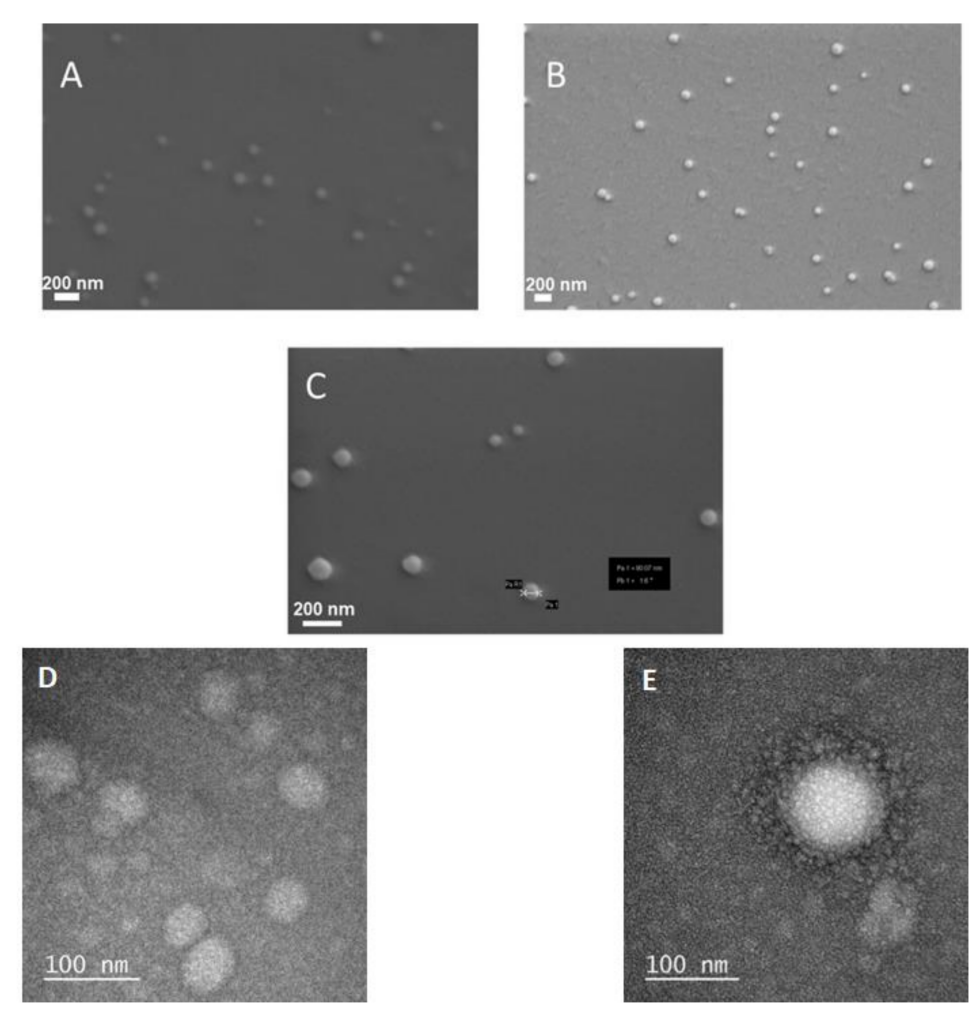
Figure 1. Electron microscopy images of different nanoparticles. FESEM image of (A) bare niosome, (B) curcumin-loaded niosomes, © chitosan-coated niosome with curcumin and Rose Bengal. TEM images of (D) bare niosome and (E) chitosan coated niosome with curcumin and Rose Bengal.
- Drug Release Studies:
- The drug release profile was assessed by placing the nanoparticles in dialysis bags, which were then immersed in PBS at different pH levels (7.4 and 5.5) and temperatures (25 °C and 37 °C).
- Samples of the release medium were taken at predetermined intervals and analyzed using UV-visible spectroscopy to quantify the amounts of curcumin and Rose Bengal released.
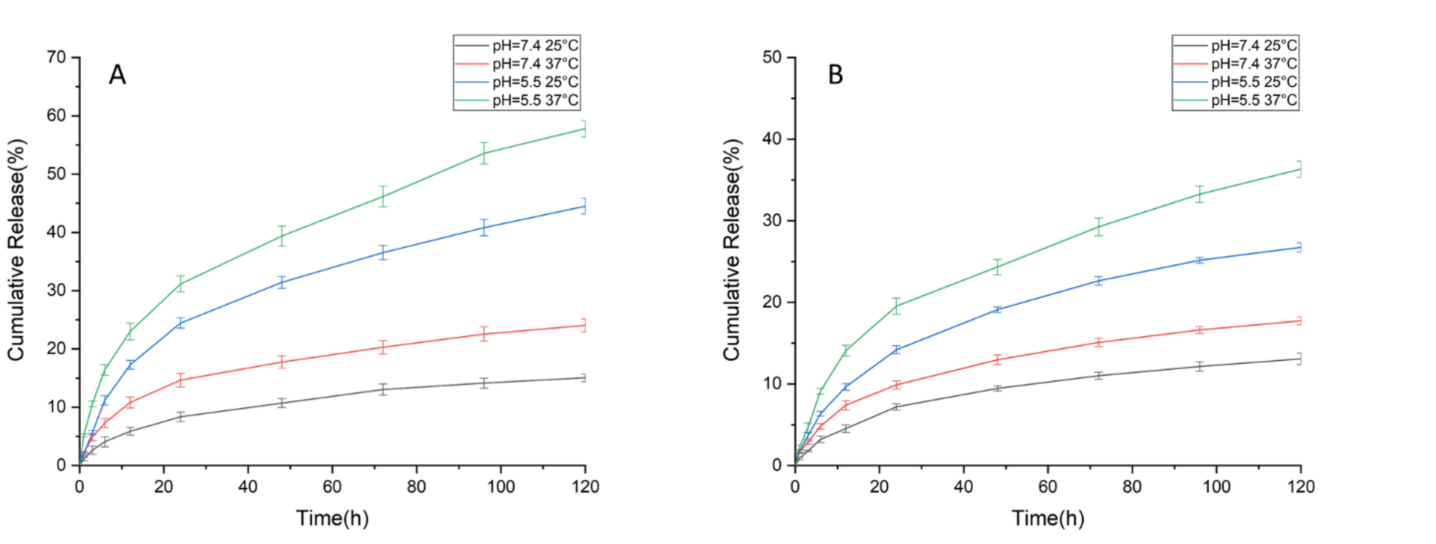
Figure 2. Drug release profile of (A) Rose Bengal and (B) CUR at two pH values and two temperatures
- Cytotoxicity Assessment:
- The anticancer efficacy of the formulation was evaluated using the MTT assay on A549 lung cancer cells. Various concentrations of the nanoparticles were tested to determine their cytotoxic effects over 24, 48, and 72 hours.
- Absorbance readings were taken to assess cell viability, allowing for a quantitative analysis of the formulation’s anticancer properties.
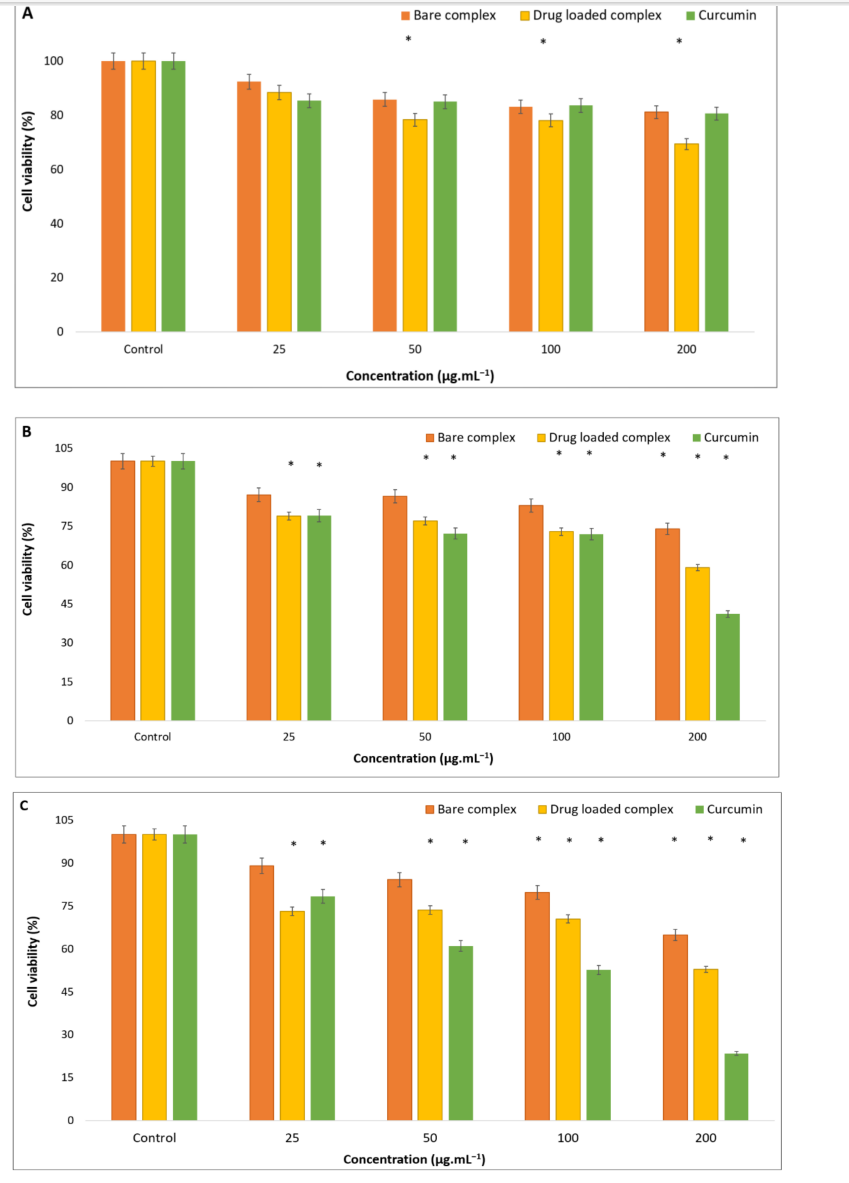
Figure 3. The results of the MTT assay test of different formulations after 24 h (A), 48 h (B), and 72 h © exposure with the A549 cancer cell line (* indicates p ≤ 0.05).
- Antibacterial Activity Testing:
- The antibacterial efficacy of the nanoparticles was tested against E. coli and S. aureus by incubating the bacteria with the nanoparticle formulations.
- Bacterial growth was assessed by counting colonies on agar plates after incubation, providing insights into the antibacterial properties of the dual-drug-loaded system.
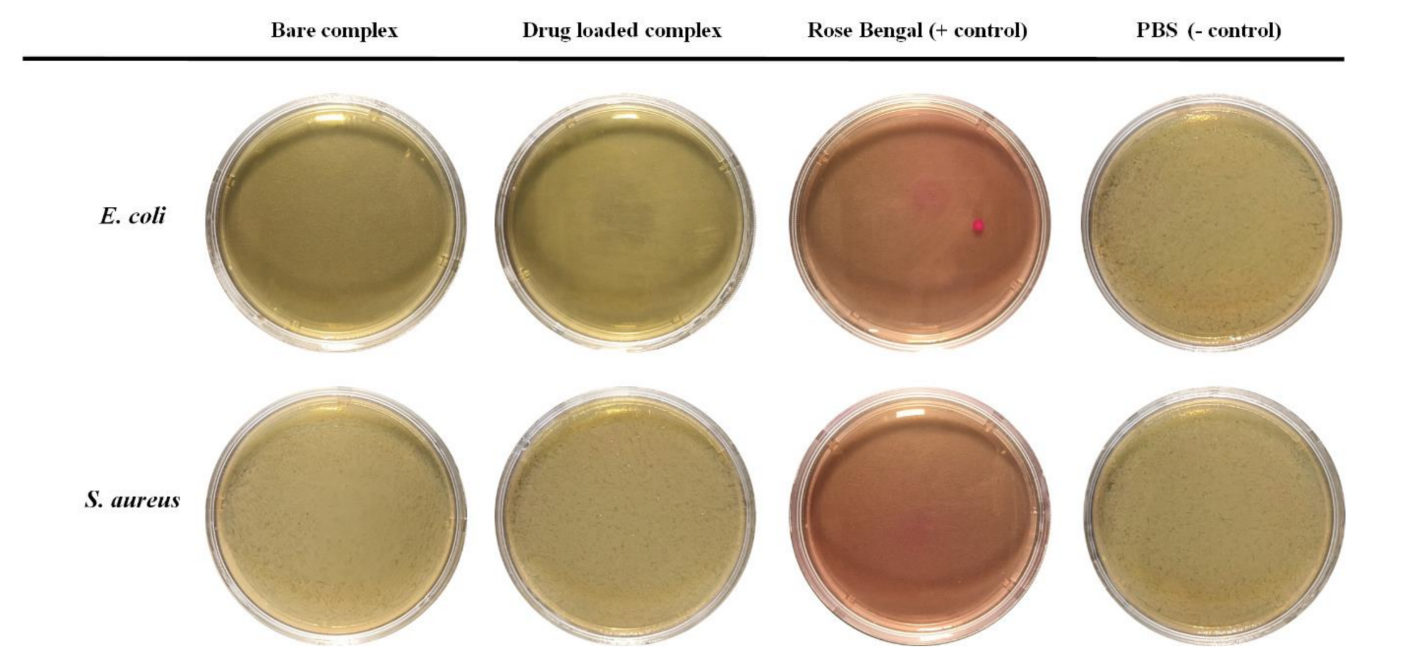
Figure 4. The results of the antibacterial test of the bare complex, drug-loaded complex, and Rose Bengal against two types of bacteria: the Gram-negative bacteria E. coli and the Gram-positive bacteria S. aureus.
Data Collection and Analysis
Data collection involved quantitative measurements from UV-visible spectroscopy for drug release studies and MTT assays for cytotoxicity assessments. The results were statistically analyzed using SPSS software, applying ANOVA tests to determine the significance of differences between experimental groups. This rigorous approach ensured the reliability and validity of the findings.
Novel Aspects
The study introduced several novel aspects, including the dual-drug loading of curcumin and Rose Bengal within a chitosan-coated niosome. This innovative platform not only enhances the bioavailability and solubility of the hydrophobic anticancer agent but also provides a targeted delivery mechanism that responds to the acidic microenvironment of cancer tissues. Compared to traditional drug delivery systems, which often suffer from premature drug release and low targeting efficiency, this approach offers improved therapeutic efficacy and the added benefit of antibacterial action, addressing the complications associated with lung cancer treatment.
Conclusion
The successful development of the dual-drug-loaded niosome-g-chitosan platform for lung cancer treatment was achieved through a meticulous process that involved the effective encapsulation of curcumin and Rose Bengal within a chitosan-coated niosome. This innovative approach not only enhanced the solubility and stability of the hydrophobic anticancer agent but also provided a targeted delivery mechanism that responds to the acidic microenvironment typical of cancer tissues. The combination of these elements resulted in a formulation that demonstrated significant cytotoxic effects against lung cancer cells while also exhibiting antibacterial properties, addressing a critical need in cancer treatment.
The highlight of the study includes the formulation’s high encapsulation efficiencies of 97% for curcumin and 98% for Rose Bengal, as well as its ability to achieve a pH and temperature-responsive drug release profile. This novel delivery system showed nearly 50% toxicity against A549 lung cancer cells over 72 hours and effective antibacterial activity against E. coli. These findings suggest that the developed platform could significantly enhance therapeutic efficacy for lung cancer while minimizing side effects, paving the way for more effective treatment strategies in the future. However, further in vivo studies are essential to confirm the efficacy and safety of this platform in clinical settings.
Reference
Zarepour, Atefeh, et al. “Fabrication of a dual-drug-loaded smart niosome-g-chitosan polymeric platform for lung cancer treatment.” Polymers 15.2 (2023): 298.
