Editor: Tiffany
Researchers have developed a novel core-shell nanoplatform, CeO₂@ZIF-8/Au, that autoregulates reactive oxygen species (ROS) to simultaneously combat bacterial infections and accelerate wound healing, offering a promising solution for infected wound management.
Key Highlights
- Research Question:
How can the balance of reactive oxygen species (ROS) be effectively regulated to enhance antibacterial efficacy and promote wound healing? - Research Difficulties:
The challenge lies in achieving a dual function—antibacterial activity through ROS generation and protective effects through ROS scavenging—without causing oxidative damage to host cells. - Key Findings:
The core-shell nanozyme, CeO2@ZIF-8/Au, demonstrates a unique ability to generate and scavenge ROS, leading to effective bacterial elimination and reduced inflammation, thereby accelerating wound healing. - Innovative Aspects:
This study introduces a spontaneous ROS balance regulator that integrates ROS generation and scavenging functions within a single nanoplatform, which is a novel approach in the field of wound healing. - Importance of the Study:
The findings could revolutionize treatment strategies for infected wounds, offering a new direction for antibacterial agents and therapies with significant implications in medical science.
The Role of Reactive Oxygen Species in Wound Healing and Infection
Wound repair is a complex biological process that becomes significantly more challenging when bacterial infections are present. Infected wounds are a pervasive issue in clinical settings, often resulting from trauma, surgery, or chronic conditions like diabetes. These wounds are characterized by symptoms such as persistent redness, swelling, pain, and the formation of pus, which signal an active infection. If left untreated, complications can escalate, including delayed wound closure, tissue necrosis, and even systemic infections that may lead to sepsis, a life-threatening condition.
The role of reactive oxygen species (ROS) in wound healing is dual-edged. At moderate levels, ROS serve as signaling molecules that help combat invading pathogens and initiate the inflammatory response necessary for healing. However, excessive ROS, often produced in infected wounds due to prolonged inflammation or bacterial activity, can cause oxidative stress, damaging healthy cells and extracellular matrix components critical for tissue regeneration. This imbalance underscores a key challenge in wound management: achieving an optimal ROS level that controls infection without impeding recovery.
Current therapeutic options for infected wounds include antibiotics, antiseptic agents, and advanced wound dressings. Antibiotics are widely used but face growing resistance from bacteria such as Escherichia coli and Staphylococcus aureus, reducing their effectiveness over time. Antiseptics, while effective against a broad spectrum of pathogens, can be cytotoxic to regenerating tissue, slowing the healing process. Advanced dressings, such as those impregnated with silver, offer some benefits but often fail to address the dynamic regulation of ROS, leaving a gap in comprehensive wound care. These limitations highlight the need for innovative strategies that both eliminate bacteria and promote tissue repair.
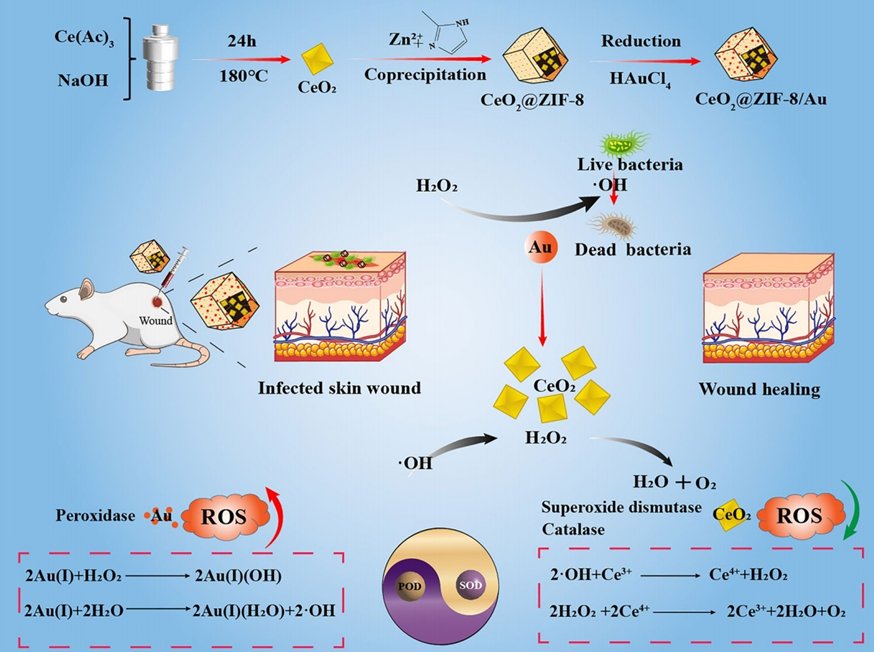
Figure 1. CeO2@ZIF-8/Au NPs treatment and healing promoting mechanism of bacterial infected wound in mice and equations of ROS production/clearance.
CeO2@ZIF-8/Au Nanoplatform for Enhanced Antibacterial Activity
The primary aim of this research was to develop a solution that balances ROS levels in infected wounds, addressing the paradox of needing ROS to fight bacteria while preventing their overaccumulation from hindering healing. The researchers hypothesized that a nanoplatform capable of autoregulating ROS—generating it to kill bacteria and then scavenging excess amounts to protect cells—could enhance wound repair. To achieve this, they designed a core-shell nanoplatform, CeO₂@ZIF-8/Au, combining cerium oxide (CeO₂) nanoparticles, a zeolitic imidazolate framework (ZIF-8), and gold (Au) nanoparticles into a single system.
This study was led by Xi Zhou and a team of collaborators from Xiamen University, along with other institutions, reflecting a multidisciplinary effort in materials science and biomedical engineering. Their findings were published in Nano-Micro Letters in 2024, marking a significant advancement in the field of nanozyme-based therapeutics.
Research Methods & Results
Experimental Process Overview
The study developed and evaluated a core-shell nanozyme, CeO2@ZIF−8/AuCeO2@ZIF−8/Au (CZA), designed to balance reactive oxygen species (ROS) for antibacterial action and wound healing. The experimental process included the following steps:
- Synthesis of CeO2CeO2 Nanoparticles: CeO2CeO2 nanoparticles were synthesized via the solvothermal method. A mixture of 0.1 M cerium acetate and 0.02 M NaOH in 80 mL ultrapure water was reacted at 180°C for 24 hours in a high-pressure vessel, then purified and dried under vacuum.
- Encapsulation in ZIF-8: CeO2CeO2 nanoparticles (35 mg) were coated with ZIF-8 to form CeO2@ZIF−8CeO2@ZIF−8 (CZ) by mixing with 1 g polyvinylpyrrolidone (PVP) in methanol, followed by 25.6 mM 2-methylimidazole and 25.2 mM zinc nitrate hexahydrate, stirred, centrifuged, and dried.
- Loading of Au Nanoparticles: Au nanoparticles were added to CZ to create CZA using a reduction method with 150 μL of 0.1 M HAuCl₄ and 3.75 mL of 0.1 M NaBH₄ in methanol, followed by purification and drying.
- Characterization: The nanoplatform’s structure and properties were confirmed using transmission electron microscopy (TEM), scanning electron microscopy (SEM), X-ray diffraction (XRD), dynamic light scattering (DLS), and Fourier transform infrared spectroscopy (FTIR).
- Antibacterial Efficacy Tests: CZA was tested in vitro against Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) using minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) assays, with and without 100 μmol/L H₂O₂.
- ROS Regulation Studies: Catalytic performance was assessed in vitro using 3,3’,5,5’-tetramethylbenzidine (TMB) as an indicator, and ROS scavenging was evaluated via electron spin resonance (ESR) spectroscopy.
- In Vivo Wound Healing: Infected wounds in BALB/c mice were treated with CZA (100 μg/mL) and 4 mM H₂O₂, with wound healing monitored over 7 days.
Key Experiments
1. Antibacterial Efficacy Test (In Vitro)
- Procedure: E. coli (ATCC25922) and S. aureus (ATCC29213) were cultured in LB broth (25 g/L, pH 4.5) with CZA concentrations ranging from 15–240 μg/mL for E. coli and 1.25–20 μg/mL for S. aureus, with or without 100 μmol/L H₂O₂. After 16 hours at 37°C, bacterial growth was measured at 595 nm (OD₅₉₅) using a microplate reader. The bacterial suspension was diluted 10⁶-fold, plated on LB agar, and colonies were counted after 16 hours (Section 2.8).
- Result: The MIC for E. coli was 120 μg/mL, and for S. aureus, it was 10 μg/mL in the presence of 100 μmol/L H₂O₂. Bacterial survival with CZA + H₂O₂ was 0% for both E. coli (at 120 μg/mL) and S. aureus (at 10 μg/mL), while other groups (PBS, H₂O₂ alone, CeO2CeO2, CZ, CZA alone) showed minimal impact on survival.
- New Finding: The combination of CZA and H₂O₂ completely eradicated both Gram-negative (E. coli) and Gram-positive (S. aureus) bacteria, highlighting CZA’s potent antibacterial efficacy due to its peroxidase-like activity generating toxic hydroxyl radicals (·OH).
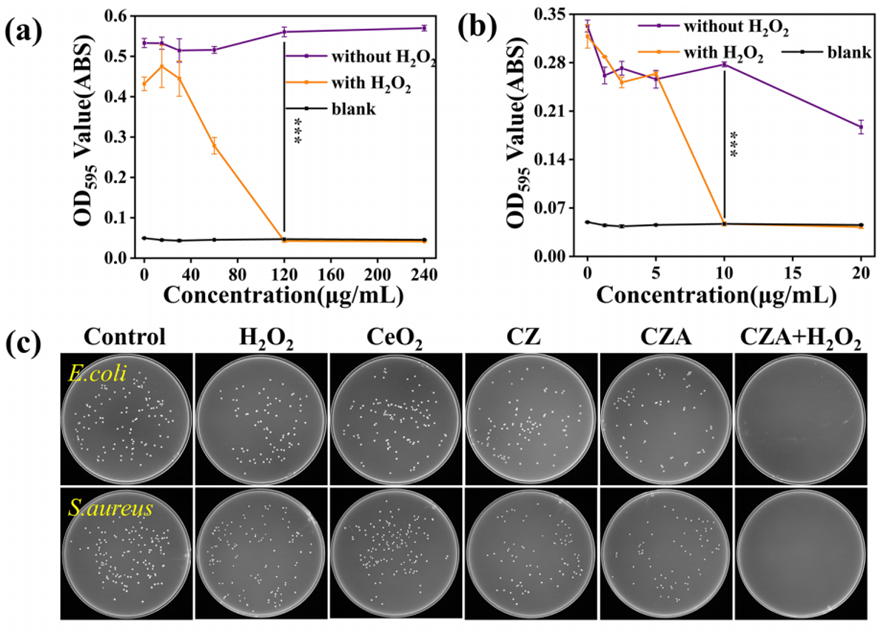
Figure 2. Characterization of antibacterial activity of materials in vitro. MIC of CZA NPs incubated with (a) E. coli and (b) S. aureus. (c) Agar plates photographs with remaining inoculated bacteria (above, E. coli; lower, S.aureus).
2. ROS Regulation Studies (In Vitro)
- Procedure: Catalytic activity was tested using 3.2 mM TMB and 500 μmol/mL H₂O₂ in a pH 4.5 NaAc buffer with 200 μg/mL CZA. UV-vis spectroscopy measured absorption at 652 nm. Steady-state kinetics were analyzed with varying H₂O₂ concentrations (5–50 mM) at pH 4.5, using Michaelis-Menten equations. ROS scavenging was assessed via ESR with 50 mM DMPO, 5 mM H₂O₂, and 200 μg/mL CZA, with ·OH generation and scavenging confirmed after acid hydrolysis (pH 4.5, 12 hours).
- Result: CZA + H₂O₂ showed strong absorption peaks at 370 and 652 nm, indicating high peroxidase-like activity. The Michaelis-Menten constant (Kₘ) was 3.69 mmol/L, and the maximum velocity (Vₘₐₓ) was 1.73 × 10⁻⁸ M/s. ESR confirmed ·OH generation by CZA and scavenging after ZIF-8 degradation, reducing ·OH peak intensity in the Fenton + CZA group.
- New Finding: CZA demonstrates a dual role: it generates ROS (·OH) for antibacterial action via Au nanoparticles and scavenges excess ROS through CeO2CeO2 release after ZIF-8 decomposition in acidic conditions, enabling autoregulation of ROS balance.
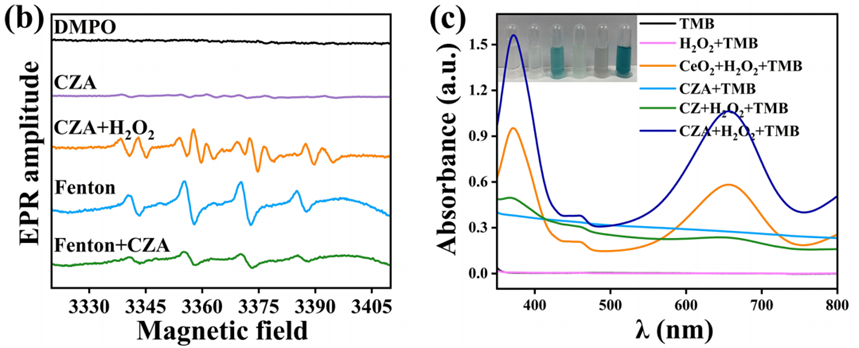
Figure 3. (b) ESR spectral of various groups. (c) UV–vis absorption of TMB in various groups (pH 4.5).
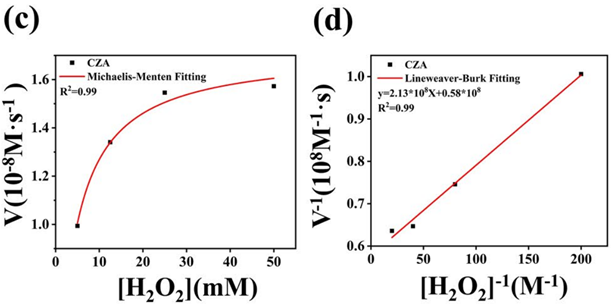
Figure 4. (c) kinetics and Lineweaver–Burk (d) curves of CZA. TEM images of CZA.
3. In Vivo Wound Healing
- Procedure: BALB/c mice (6–8 weeks old) were injected subcutaneously with 200 μL of 10⁷ CFU/mL S. aureus. After 24 hours, a 7 mm wound was created at the injection site. Mice were divided into seven groups (n=3 each) and treated with 20 μL of PBS (control), H₂O₂ (4 mM), CeO2CeO2 (100 μg/mL), CZ (100 μg/mL), CZA (100 μg/mL), ZIF-8/Au (ZA) + H₂O₂, or CZA (100 μg/mL) + 4 mM H₂O₂. Wound size was measured every other day for 7 days using ImageJ, and tissues were analyzed via H&E staining.
- Result: On day 7, the wound area in the CZA + H₂O₂ group was reduced to 7.5%, compared to 23.8% in the PBS group, 22.8% with H₂O₂, 23.1% with CeO2CeO2, 25.3% with CZ, 23.6% with CZA alone, and 15.6% with ZA + H₂O₂. H&E staining showed improved tissue regeneration and reduced inflammation in the CZA + H₂O₂ group.
- New Finding: CZA + H₂O₂ accelerated wound healing, achieving a 92.5% reduction in wound area by day 7, significantly outperforming other treatments. This reflects its combined antibacterial action and ROS scavenging, promoting tissue repair in infected wounds.
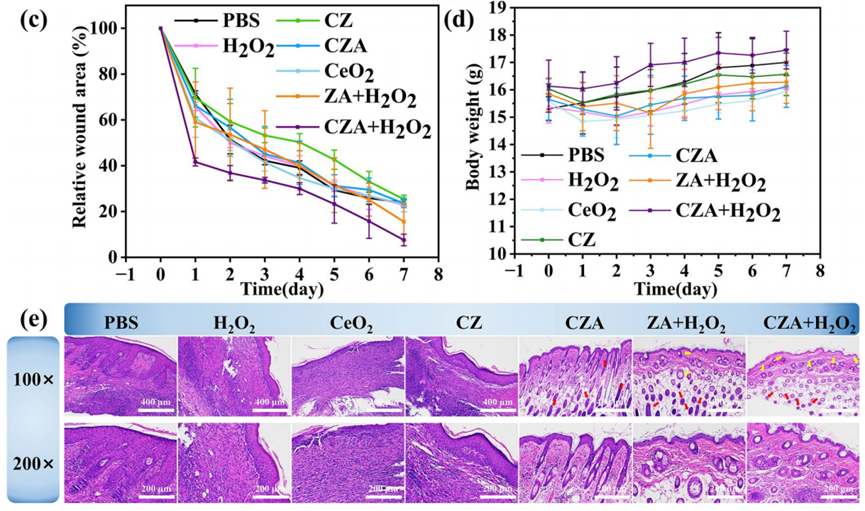
Figure 5. (c) Corresponding changes in relative wound area. (d) Mice body weight. (e) H&E stained photographs at wound site (gules and yellow arrows mean hair follicles and blood vessels).
Implications for Future Applications in Wound Healing
This study demonstrates that the CeO₂@ZIF-8/Au nanoplatform effectively autoregulates ROS levels, offering a dual-action approach to wound healing. Initially, it generates ROS to eliminate bacterial pathogens, leveraging the peroxidase-like activity of Au nanoparticles and the zinc ions from ZIF-8 degradation. Subsequently, the CeO₂ core scavenges excess ROS, mitigating oxidative stress and fostering an environment conducive to tissue repair. The results from both in vitro and in vivo experiments underscore its high antibacterial efficiency and healing promotion.
The novelty of this approach lies in its spontaneous, environment-responsive functionality, eliminating the need for external triggers or multiple agents. By integrating ROS generation and scavenging into a single system, the nanoplatform overcomes the shortcomings of conventional therapies. Its success in preclinical models suggests potential for clinical translation, not only for wound care but also for other conditions involving ROS dysregulation, such as chronic inflammation or ischemic injuries. This research paves the way for next-generation nanomaterials in biomedical applications, offering hope for improved outcomes in infection-related wound management.
Reference:
Zhou, Xi, et al. “ROS balance autoregulating core–shell CeO2@ ZIF-8/Au nanoplatform for wound repair.” Nano-Micro Letters 16.1 (2024): 156.
