Scientists develop a bacterial protoplast-derived nanovesicle system to deliver CRISPR-Cas9 tools targeting tumor-associated macrophages for improved cancer immunotherapy.
Key Preview
Research Question
Recent advancements in cancer treatment using CRISPR-Cas9 technology show promise in gene editing to target tumor-associated macrophages (TAMs). However, significant challenges remain, including maintaining cell viability post-editing and ensuring effective in vivo delivery. This study investigates the development of a novel in vivo CRISPR-Cas9 system utilizing bacterial protoplast-derived nanovesicles to specifically target TAMs for enhanced cancer immunotherapy.
Research Design and Strategy
The research employs a systematic design, utilizing bacterial protoplast-derived nanovesicles (NVs) engineered with pH-responsive PEG-conjugated phospholipids and galactosamine-conjugated phospholipids. The goal is to facilitate targeted delivery of CRISPR-Cas9 tools to re-educate TAMs, thus reshaping the tumor microenvironment.
Method
The team utilized plasmid-transformed E. coli protoplasts to produce NVs loaded with Cas9-sgRNA ribonucleoproteins targeting the Pik3cg gene, known for its role in macrophage polarization. Additionally, CpG-rich DNA fragments were included as potent TLR9 ligands to stimulate the immune response. The efficacy of these NVs was tested in female mice with tumor models to observe their impact on tumor growth and macrophage polarization.
Key Results
The study yielded significant results, demonstrating that the NVs effectively targeted TAMs, promoting an anti-tumor M1-like phenotype. This transformation resulted in substantial tumor growth inhibition in mice models. The nanovesicles exhibited high encapsulation efficiency of CRISPR components, ensuring precise gene delivery without compromising the viability of the macrophages, in contrast to prior methods that posed risks of toxicity and reduced efficacy.
Significance of the Research
This research marks a pivotal advancement in cancer immunotherapy by addressing critical challenges associated with CRISPR-Cas9 delivery. The use of bacterial-derived NVs not only enhances the safety profile of gene editing tools but also provides a scalable method for clinical applications. The findings suggest that this innovative approach could lead to more effective cancer treatments, particularly by modifying the behavior of TAMs within the tumor microenvironment.
Introduction
Cancer remains one of the leading causes of morbidity and mortality worldwide, characterized by the uncontrolled growth and spread of abnormal cells. Among the various types of cancer, breast cancer, melanoma, and other solid tumors are particularly challenging due to their complex tumor microenvironments and the presence of tumor-associated macrophages (TAMs), which often contribute to tumor progression and immune evasion. Traditional cancer treatment strategies typically involve chemotherapy, radiation therapy, or targeted therapies, which aim to eliminate cancer cells. However, these approaches often face significant obstacles, particularly in effectively delivering therapeutic agents to the tumor site.
Common strategies for drug delivery in traditional cancer treatments rely on systemic administration of chemotherapeutics or biologics. These methods typically result in the distribution of drugs throughout the body, which not only reduces the concentration of the drug at the tumor site but also exposes healthy tissues to potentially toxic effects. This generalized delivery can lead to insufficient therapeutic response, resulting in treatment resistance, recurrence of the disease, and significant side effects that diminish the quality of life for patients.
The current challenges in drug delivery for cancer therapies stem from the difficulty in achieving targeted and effective delivery of therapeutic agents to tumor cells while sparing healthy tissues. Factors such as the heterogeneity of tumors, the presence of biological barriers, and the immunosuppressive microenvironment contribute to the suboptimal performance of conventional delivery systems. Consequently, many patients experience limited efficacy from their treatments and may suffer from adverse reactions.
To address these challenges, innovative drug delivery strategies are being developed. One promising approach involves the use of engineered nanovesicles derived from bacterial protoplasts, designed to encapsulate therapeutic agents such as CRISPR-Cas9 systems. This novel delivery method enhances targeting specificity to TAMs, which play a crucial role in the tumor microenvironment. By utilizing pH-responsive and ligand-targeted nanovesicles, this strategy aims to improve the precision of drug delivery, increase therapeutic efficacy, and minimize systemic side effects, paving the way for more effective cancer immunotherapy.
Research Team and Aim
The research was conducted by a collaborative team of scientists led by Dr. Junfeng Zhang from Nanjing University. The study took place over the course of several years, culminating in the publication of their findings in early 2024. The paper is titled “Bacterial protoplast-derived nanovesicles carrying CRISPR-Cas9 tools re-educate tumor-associated macrophages for enhanced cancer immunotherapy” and was published in Nature Communications.
The primary aim of the research, as articulated by Dr. Zhang, was to leverage CRISPR-Cas9 technology to modify tumor-associated macrophages (TAMs) within the tumor microenvironment. The goal was to inhibit tumor growth by reprogramming these macrophages into an anti-tumor M1-like phenotype, thereby enhancing the overall efficacy of cancer immunotherapy while addressing significant challenges related to the safe and effective delivery of gene-editing tools in vivo.
Experimental Process
Primary Technique
The primary technique utilized in this research is the development and application of bacterial protoplast-derived nanovesicles (NVs) for targeted delivery of CRISPR-Cas9 gene editing tools to tumor-associated macrophages (TAMs). This innovative approach combines the advantages of bacterial-derived NVs with CRISPR-Cas9 technology to enhance cancer immunotherapy.
Key Steps
- Protoplast Preparation:
- E. coli carrying plasmids for Cas9 and sgRNA targeting the Pik3cg gene were cultured until the desired optical density was reached.
- The bacteria were treated with lysozyme to create protoplasts by removing the outer membrane, which is essential for reducing toxicity in subsequent steps.
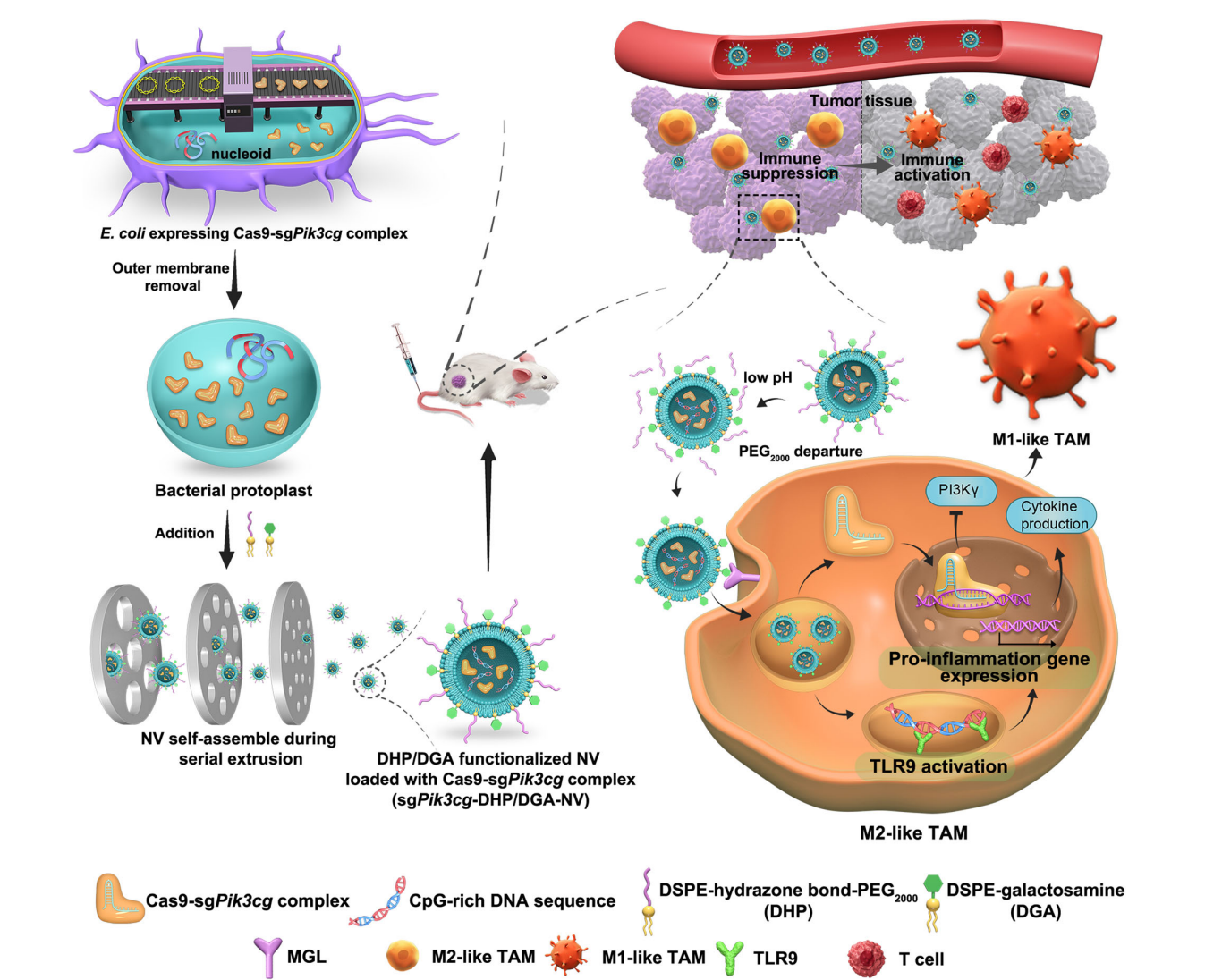
Figure 1. Schematic design of E. coli protoplast-derived nanovesicles (sgPik3cgDHP/DGA-NVs) for TAM-selective genome editing to enhance anti-tumor efficacy.
- Nanovesicle Formation:
- The protoplasts were subsequently extruded in the presence of pH-responsive phospholipid derivatives (DHP) and TAM-targeting phospholipids (DGA).
- This process led to the self-assembly of NVs encapsulating the Cas9-sgRNA ribonucleoprotein and CpG-rich DNA fragments, resulting in a highly efficient nanovesicle formation.
- Characterization of NVs:
- The size, morphology, and encapsulation efficiency of the NVs were analyzed using transmission electron microscopy (TEM), nanoparticle tracking analysis (NTA), and dynamic light scattering (DLS).
- The stability of the NVs under various conditions was assessed, confirming their robustness for in vivo applications.
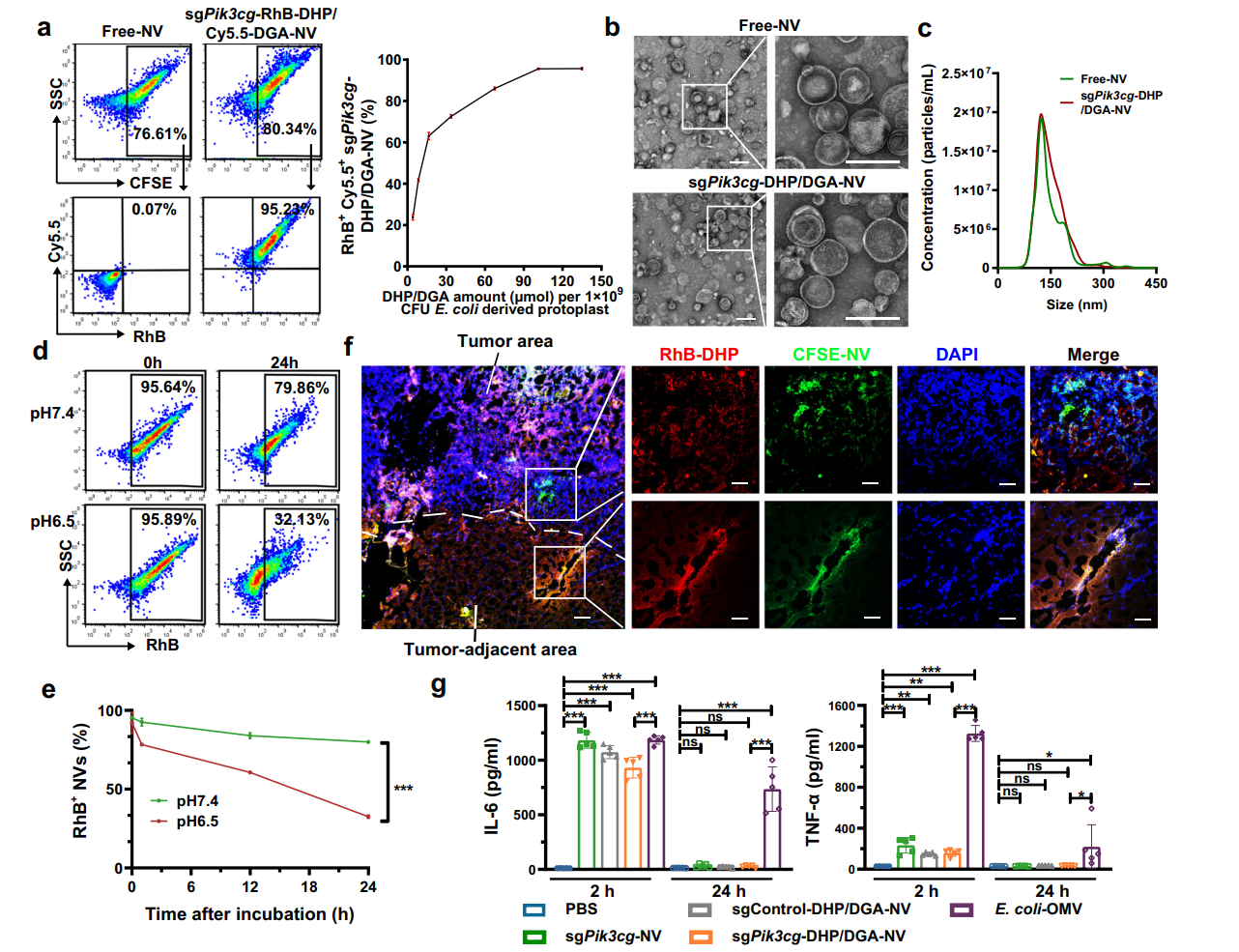
Figure 2. The preparation and characterization of sgPik3cg-DHP/DGA-NVs
- In Vitro Uptake Studies:
- The efficacy of NVs in targeting M2-like macrophages was evaluated by incubating the cells with fluorescently labeled NVs.
- Flow cytometry and microscopy were employed to quantify the uptake efficiency, demonstrating the specific binding of DGA-functionalized NVs to macrophages expressing the galactose-type lectin receptor.
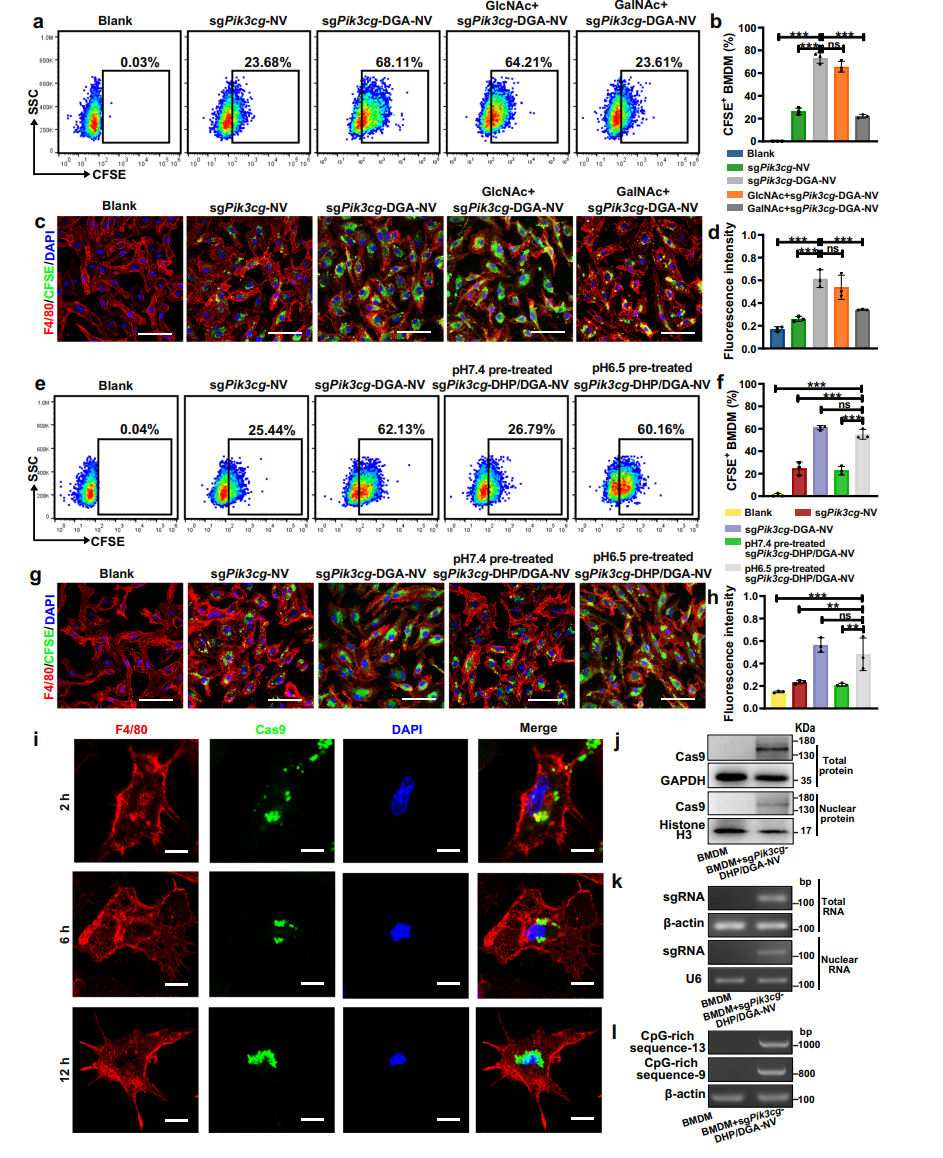
Figure 3. In vitro uptake of NVs by M2-like macrophages via MGL-mediated endocytosis
- In Vivo Delivery and Efficacy Assessment:
- The NVs were intravenously injected into tumor-bearing mice to assess their biodistribution and therapeutic effects.
- Fluorescence imaging and flow cytometry were used to track the accumulation of NVs in TAMs and to evaluate gene editing efficiency in vivo, including the monitoring of tumor size and immune response.
Data Collection and Analysis
Data were collected through a combination of fluorescence imaging, flow cytometry, and biochemical assays. Quantitative analysis was performed using statistical methods to assess differences in treatment effects, including tumor growth inhibition and immune marker expression. This rigorous data collection process ensured the reliability and validity of the results.
Novel Aspects
The study’s novel aspects include the use of engineered bacterial protoplasts to produce NVs with high encapsulation efficiency and targeted delivery capabilities. This method overcomes the limitations of traditional nano-delivery systems by enhancing the precision of gene editing while maintaining the viability of TAMs. The ability to reprogram TAMs into an anti-tumor phenotype in a single step presents a significant advancement in cancer immunotherapy strategies, potentially leading to more effective treatments with fewer side effects compared to conventional methods.
Conclusion
The successful development of this innovative drug delivery system was achieved through the meticulous engineering of bacterial protoplast-derived nanovesicles (NVs) that effectively encapsulate CRISPR-Cas9 tools. By employing a systematic approach that involved the preparation of E. coli protoplasts, the incorporation of pH-responsive and TAM-targeting phospholipids, and the optimization of encapsulation techniques, the researchers were able to enhance the targeted delivery of gene-editing components specifically to tumor-associated macrophages (TAMs).
A highlight of the study is the demonstration that the NVs not only effectively targeted and reprogrammed TAMs into an anti-tumor M1-like phenotype but also maintained their viability and functionality within the hostile tumor microenvironment. This dual capability is critical, as it addresses one of the significant challenges of traditional cancer therapies—ensuring precise delivery of therapeutic agents while minimizing off-target effects and systemic toxicity. Overall, these findings suggest that this novel NV-based delivery system holds great promise for improving the efficacy and safety of cancer immunotherapy, paving the way for future clinical applications.
Reference:
Here is the MLA format reference for the paper: Zhao, Mingming, et al. “Bacterial protoplast-derived nanovesicles carrying CRISPR-Cas9 tools re-educate tumor-associated macrophages for enhanced cancer immunotherapy.” Nature Communications, vol. 15, no. 950, 2024.
