Scientists formulate Zerumbone-loaded liquid crystalline nanoparticles that significantly inhibit the proliferation and migration of non-small-cell lung cancer cells, showcasing enhanced therapeutic efficacy compared to free Zerumbone.
Key Preview
- Research Question: This study aims to address the challenges associated with the low solubility and bioavailability of Zerumbone, a promising anti-cancer compound, and to evaluate the efficacy of a novel formulation—Zerumbone-loaded liquid crystalline nanoparticles (ZER-LCNs)—in inhibiting the proliferation and migration of non-small-cell lung cancer (NSCLC) cells.
- Research Design and Strategy: The research employs an experimental design focusing on in vitro assays with A549 lung cancer cells to assess the anti-cancer properties of ZER-LCNs compared to free Zerumbone, thereby providing insights into the potential of this nanoparticle formulation as an effective treatment strategy for NSCLC.
- Method: The study utilizes an ultrasonication method to formulate ZER-LCNs, followed by in vitro assays such as the MTT assay for cell proliferation, colony formation assays, and wound healing assays to evaluate the effects of the nanoparticles on cancer cell behavior.
- Key Results: The results demonstrate that ZER-LCNs significantly inhibit A549 cell proliferation by approximately 90% at doses of 7.5 and 10 µM, and reduce cell migration by around 60% compared to controls, highlighting their superior efficacy over free Zerumbone.
- Significance of the Research: This research is significant as it provides a novel approach to enhance the therapeutic potential of Zerumbone through nanotechnology, potentially leading to more effective treatment options for lung cancer patients while minimizing adverse effects associated with traditional therapies.
Introduction
Lung cancer, particularly non-small-cell lung cancer (NSCLC), is one of the most prevalent and lethal forms of cancer worldwide, accounting for a significant proportion of cancer-related deaths. It poses a serious public health challenge due to its often late diagnosis and aggressive nature. Traditional treatment strategies for NSCLC typically involve surgical resection, chemotherapy, and radiation therapy, which aim to eliminate cancer cells and reduce tumor size. However, these conventional approaches frequently rely on systemic drug delivery methods that can lead to inadequate targeting of cancer cells, resulting in suboptimal therapeutic outcomes.
One of the key challenges associated with traditional drug delivery methods is the development of drug resistance, which can occur due to the heterogeneity of tumor cells and their ability to adapt to therapy. Additionally, patients often experience significant adverse effects from these treatments, which can severely impact their quality of life. The limitations of conventional drug delivery systems, including poor solubility and bioavailability of certain therapeutic agents, further complicate the effectiveness of treatment regimens.
To address these challenges, innovative drug delivery strategies utilizing nanotechnology have emerged. These approaches, particularly the formulation of bioactive compounds into nanoparticles, enhance drug solubility, stability, and targeting capabilities. Liquid crystalline nanoparticles (LCNs) represent a promising advancement in drug delivery systems, offering the potential for controlled release and improved bioavailability of therapeutic agents. By encapsulating Zerumbone, a natural compound with demonstrated anti-cancer properties, into LCNs, this study aims to leverage these advancements to enhance the therapeutic efficacy against NSCLC while minimizing the associated side effects of conventional treatments.
Research Team and Aim
The research team is comprised of experts in the fields of pharmacology and cancer research, led by Dr. Keshav Raj Paudel from the University of Technology Sydney. This study was conducted in 2023 and culminated in the publication of the paper titled “Zerumbone-incorporated liquid crystalline nanoparticles inhibit proliferation and migration of non-small-cell lung cancer in vitro” in the journal Naunyn-Schmiedeberg’s Archives of Pharmacology. The aim of the research, as articulated by Dr. Paudel, is to “formulate Zerumbone-loaded liquid crystalline nanoparticles and investigate their effectiveness against non-small-cell lung cancer in vitro using A549 lung cancer cells.” This study seeks to evaluate the anti-cancer potential of the novel formulation while addressing the limitations associated with the solubility and bioavailability of Zerumbone.
Experimental Process
Primary Technique
The primary technique employed in this study is the formulation of Zerumbone-loaded liquid crystalline nanoparticles (ZER-LCNs) using an ultrasonication method. This approach not only enhances the solubility and bioavailability of Zerumbone, a naturally occurring compound with anti-cancer properties, but also facilitates targeted delivery to lung cancer cells.
Key Steps
- Formulation and Characterization of ZER-LCNs: The formulation process began with the heating of monoolein (MO) to 60 °C, followed by the incorporation of Zerumbone into the molten MO. This mixture was then combined with Poloxamer 407 dissolved in water, creating a coarse dispersion. The dispersion was subsequently subjected to ultrasonication for size reduction, resulting in ZER-LCNs characterized by a mean diameter of approximately 180.6 nm and an entrapment efficiency of 90.63%.
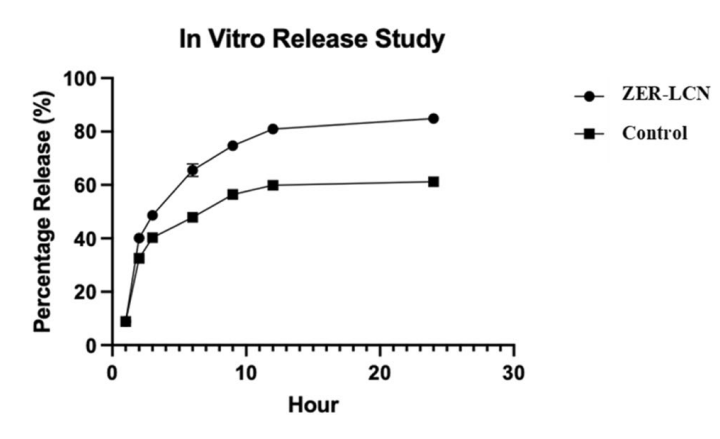
Figure 1. The in vitro release study of free ZER (control) and ZERLCNs
- In Vitro Biological Activity Assessment: A549 lung cancer cells were treated with varying concentrations of ZER-LCNs and free Zerumbone for 24 hours. Cell proliferation was measured using the MTT assay, which quantifies viable cells based on their ability to convert MTT into formazan crystals. The results indicated a dose-dependent inhibition of cell proliferation, with ZER-LCNs demonstrating significantly greater efficacy at lower concentrations compared to free Zerumbone.
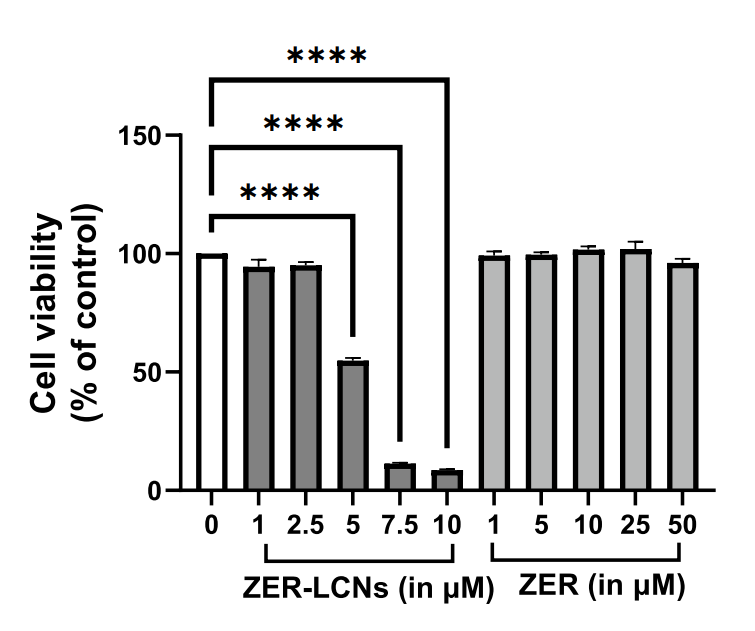
Figure 2. Anti-proliferative efects of ZER and ZER-LCNs in A549 cells. A549 cells were treated with or without ZER-LCNs (1, 2.5, 5, 7.5, or 10 µM) or ZER (1, 5, 10, 25, or 50 µM) for 24 h, followed by incubation with MTT. The purple formazan crystals formed were dissolved with DMSO and the absorbance was measured with microplate reader. The data in the fgure are mean ± SEM of 3 independent experiments. ****p<0.0001 - Colony Formation and Migration Assays: The colony formation assay involved plating A549 cells and treating them with either ZER or ZER-LCNs. After two weeks of incubation, the colonies were stained with crystal violet to visualize and quantify the number of colonies formed. Additionally, the wound healing assay assessed the migration capacity of A549 cells by creating a scratch in a cell monolayer and measuring the rate of wound closure over 24 hours.
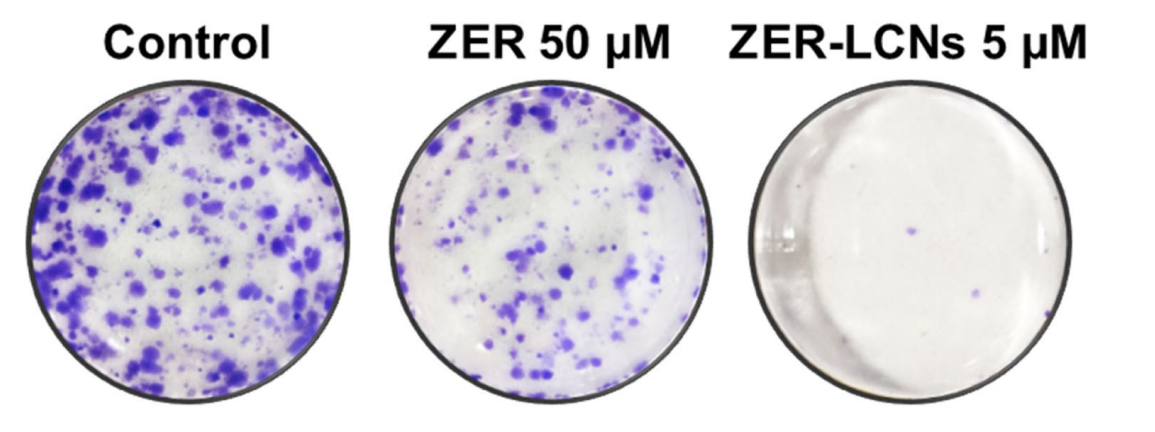
Figure 3. Efect of ZER and ZERLCNs on colony formation of A549 cells. After seeding A549 cells in 6-well plate, they were cultured for 2 weeks in the absence or presence of 50 µM ZER or 5 µM ZER-LCNs. The cell colonies were stained with crystal violet and photographed. The fgure shows representative images from 3 independent experiments
- Molecular Mechanism Investigation: Reverse transcriptase-quantitative PCR (RT-qPCR) was utilized to analyze the expression levels of key tumor suppressor genes (P53 and PTEN) and the metastasis-associated gene (KRT18) in A549 cells treated with ZER-LCNs. Protein expression was further evaluated using a proteome profiler human oncology array, which allowed for the assessment of several proteins associated with cancer proliferation and migration.
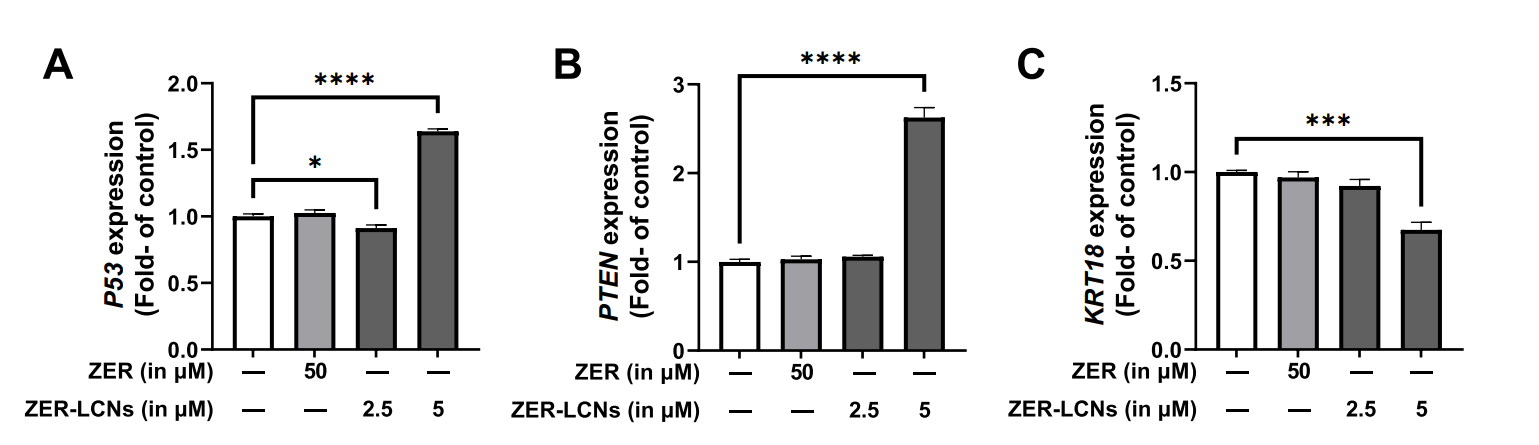
Figure 4. Regulation of mRNA levels P53, PTEN, and KRT18 by ZER and ZER-LCNs. A549 cells were treated with 50 µM ZER, 2.5 µM ZER-LCNs, or 5 µM ZER-LCNs for 24 h. Total RNA was extracted, cDNA synthesized, and mRNA levels were determined with qPCR. The fgure shows the mRNA levels of P53 (A), PTEN (B) and KRT18 ©, normalized against the levels of GAPDH. Data are expressed as mean ±SEM of 3 independent experiments. *p<0.05, ***p<0.001, ****p<0.0001
Data Collection and Analysis
Data collection involved measuring absorbance at specific wavelengths using UV-Vis spectrophotometry to assess drug concentration in various samples. Statistical analyses were conducted using one-way ANOVA, followed by Dunnett’s multiple comparison tests to evaluate the significance of the results. This rigorous approach ensured reliable and reproducible findings.
Novel Aspects
This study highlights several novel aspects of the ZER-LCN formulation. Firstly, the use of liquid crystalline nanoparticles allows for improved drug solubility and a controlled release profile, addressing the limitations of traditional nano-delivery systems that often struggle with drug stability and bioavailability. Additionally, the significant enhancement of anti-cancer efficacy at lower doses of ZER-LCNs compared to free Zerumbone showcases the potential for this innovative approach to provide more effective treatment options with reduced side effects for lung cancer patients. This advancement in nanotechnology could pave the way for further research and development in targeted cancer therapies.
Conclusion
The successful development of the Zerumbone-loaded liquid crystalline nanoparticles (ZER-LCNs) as a drug delivery system has been achieved through careful formulation and characterization techniques, notably the ultrasonication method, which ensures enhanced solubility and bioavailability of Zerumbone. The study demonstrated that ZER-LCNs significantly inhibit the proliferation and migration of A549 lung cancer cells, showcasing a remarkable anti-cancer potency at much lower concentrations compared to free Zerumbone. The highlight of this study lies in the demonstration that ZER-LCNs can effectively enhance the therapeutic efficacy of Zerumbone against non-small-cell lung cancer, addressing critical challenges such as poor drug solubility and bioavailability. By leveraging nanotechnology, this innovative approach not only provides a promising treatment strategy for lung cancer but also paves the way for further research into targeted therapies utilizing similar drug delivery systems.
