This study investigates the development of paclitaxel-loaded polymeric nanoparticles (PLGA NPs) for effective brain delivery, focusing on their application in glioblastoma treatment through both intravenous and intranasal administration routes.
Key Preview
Research Question:
The study addresses the challenge of delivering paclitaxel (PTX) effectively to the brain to treat glioblastoma, considering the blood-brain barrier (BBB) as a significant obstacle for conventional treatments.
Research Design and Strategy:
The study utilizes polymeric nanoparticles (PLGA NPs) loaded with paclitaxel and explores two delivery methods—intranasal (IN) and intravenous (IV)—to compare their effectiveness in delivering paclitaxel to brain tissues while avoiding systemic toxicity.
Method:
The research uses a combination of in vitro and in vivo experiments. In vitro, an MTT cell proliferation assay on glioblastoma cells (U87MG) is used to assess the anti-proliferative effect of PTX-loaded PLGA NPs. In vivo, male Sprague-Dawley rats are used to study the pharmacokinetics and biodistribution of PTX-loaded PLGA NPs administered via IN and IV routes.
Key Results:
The results showed that both IN and IV administration of PTX-loaded PLGA NPs led to significant accumulation of paclitaxel in brain tissues, with no signs of toxicity. The nanoparticles demonstrated comparable anti-proliferative effects on glioblastoma cells as free paclitaxel, and their controlled release resulted in improved bioavailability and brain delivery.
Significance of the Research:
This research is significant as it demonstrates the potential of using polymeric nanoparticles for non-invasive delivery of chemotherapy drugs like paclitaxel to the brain, offering an alternative to conventional treatments that cannot effectively cross the blood-brain barrier.
Introduction
Research into brain tumor treatments, especially glioblastoma, has faced significant challenges due to the difficulty of drugs crossing the blood-brain barrier (BBB). Current treatments often have limited efficacy due to this barrier and systemic side effects. This paper innovates by using PLGA nanoparticles, which are biodegradable and have shown promising results for drug delivery. The current research aims to improve the delivery of paclitaxel, a widely used anti-cancer drug, to the brain, exploring both intravenous and intranasal delivery routes. This study is necessary to enhance the efficacy of glioblastoma treatments by developing a more effective method for drug delivery to the brain while minimizing toxicity.
Research Team and Objective
The research team consists of Muhammad AbdEl-haq, Awanish Kumar, Fatima-ezzahra Ait Mohand, Nataly Kravchenko-Balasha, Yakir Rottenberg, and Abraham J. Domb from the Hebrew University of Jerusalem, Israel. The paper is titled Paclitaxel Delivery to the Brain for Glioblastoma Treatment and was published in the International Journal of Molecular Sciences in 2023. The study’s primary objective is to enhance the delivery of paclitaxel to the brain through the use of PLGA nanoparticles, comparing intranasal and intravenous administration methods. A key innovation of this research is the development of a drug delivery system that improves the bioavailability of paclitaxel in the brain while avoiding the toxicity typically associated with intravenous administration.
Experimental Process
- Preparation of PTX-Loaded PLGA Nanoparticles
Key Steps: The PTX-loaded PLGA nanoparticles were prepared using a solvent evaporation method. In this process, 50 mg of paclitaxel (PTX) and 500 mg of PLGA (poly(lactic-co-glycolic acid)) were dissolved in 10 mL of acetonitrile. This organic solution was added dropwise into a 50 mL aqueous solution containing 0.2% w/v of the non-ionic surfactant Solutol HS15, followed by stirring at 1000 rpm for 1 hour. After the solvent evaporation step using a rotavapor, the nanoparticles were collected by centrifugation at 7000 rpm for 50 minutes and washed twice to remove excess surfactant.
Result and Key Data: The resulting nanoparticles had a drug loading of 9-10% w/w and a loading efficiency of approximately 98%. The particle size was determined to be 216 nm by Dynamic Light Scattering (DLS) and 191.9 nm by Transmission Electron Microscopy (TEM). The nanoparticles exhibited spherical morphology and a surface charge of -16 mV.
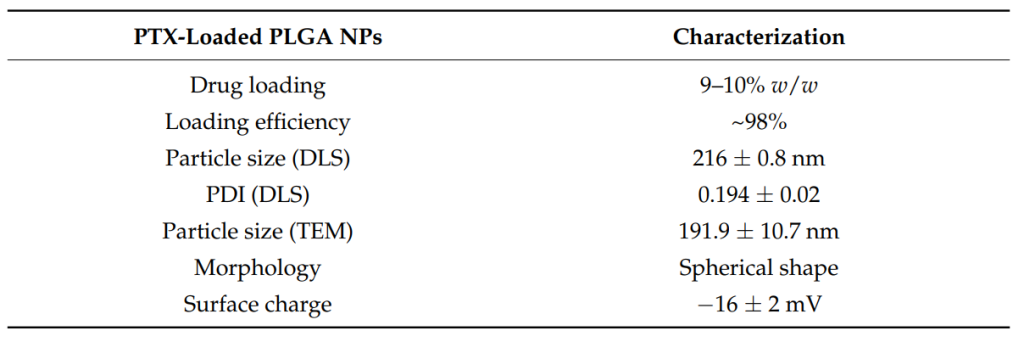
Figure 1. Physical characterization of the PLGA NPs.
Significance of the Result: The successful preparation of PTX-loaded nanoparticles with controlled particle size and high drug loading is crucial for ensuring efficient drug delivery to the brain. The particle size (216 nm) falls within the optimal range for avoiding mucociliary clearance in the nasal cavity and ensuring good uptake through the blood-brain barrier (BBB).
Key Innovations: The incorporation of Solutol HS15 as a surfactant enhanced the stability and bioavailability of the PTX-loaded PLGA nanoparticles, facilitating their use in non-invasive delivery methods such as intranasal administration.
2. In Vitro Drug Release Study
Key Steps: To evaluate the release profile of paclitaxel from the PLGA nanoparticles, the drug release study was conducted in a phosphate-buffered saline (PBS) solution containing 1% Tween 80. A total of 10 mg of PTX-loaded nanoparticles was placed in an Eppendorf vial with 2 mL of the release medium, and the system was kept at 37°C with continuous shaking at 75 rpm. The release was monitored over 20 days.
Result and Key Data: The study found that approximately 30% of PTX was released from the nanoparticles over the 20-day period, consistent with the known slow-release characteristics of PLGA-based systems. The release was gradual, indicating a controlled release mechanism that is essential for sustained therapeutic effects.
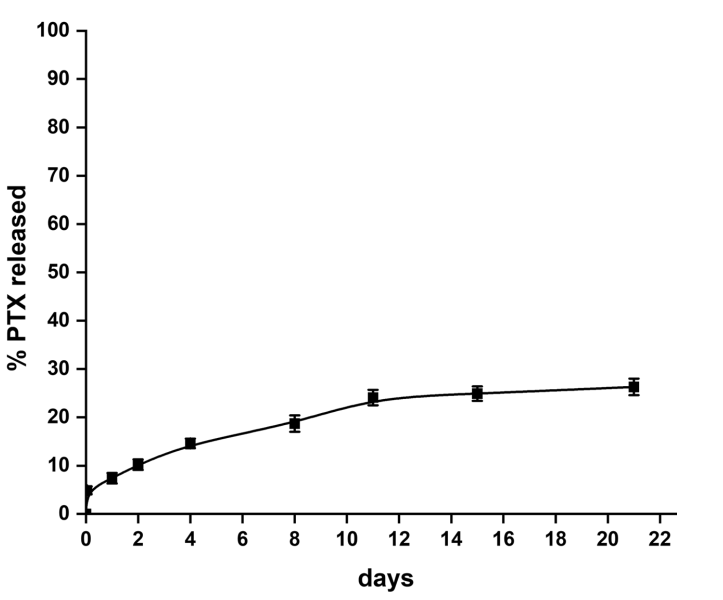
Figure 2. Release of PTX from the PLGA NPs in 1% Tween 80 at 37 °C
Significance of the Result: The controlled and sustained release of paclitaxel from the PLGA nanoparticles is critical for maximizing the drug’s therapeutic efficacy while minimizing side effects associated with peak plasma concentrations. This release profile also suggests that the nanoparticles can maintain a prolonged presence at the target site, enhancing drug delivery to glioblastoma cells over an extended period.
Key Innovations: The use of PLGA nanoparticles for controlled drug release in glioblastoma therapy offers a significant innovation, as it enables precise and sustained delivery of paclitaxel to the brain, overcoming issues of rapid drug metabolism and clearance often seen with conventional formulations.
3. In Vivo Pharmacokinetics and Biodistribution Study
Key Steps: Male Sprague-Dawley rats were divided into two groups, receiving PTX-loaded PLGA nanoparticles either via intravenous (IV) or intranasal (IN) administration at a dose of 5 mg/kg. Blood samples were collected at various time points (0, 5, 15, 30, 60, 120, 180, 240, 360, and 480 minutes) after administration to assess the pharmacokinetic parameters of PTX. Additionally, the rats’ brain, liver, and kidney tissues were harvested at different time points for biodistribution analysis.
Result and Key Data: After both IV and IN administration, PTX was rapidly absorbed into the bloodstream, with the peak plasma concentration (Cmax) observed within 5 minutes of administration. The Area Under the Curve (AUC) values for the IV group were higher in the liver and kidney tissues, indicating that these organs played a larger role in metabolizing the drug. Brain accumulation was significant for both administration routes, although the concentration in the brain was slightly higher after intravenous administration (AUC0–24 of 4.5 ng·h/mL) compared to intranasal administration (AUC0–24 of 2.0 ng·h/mL).
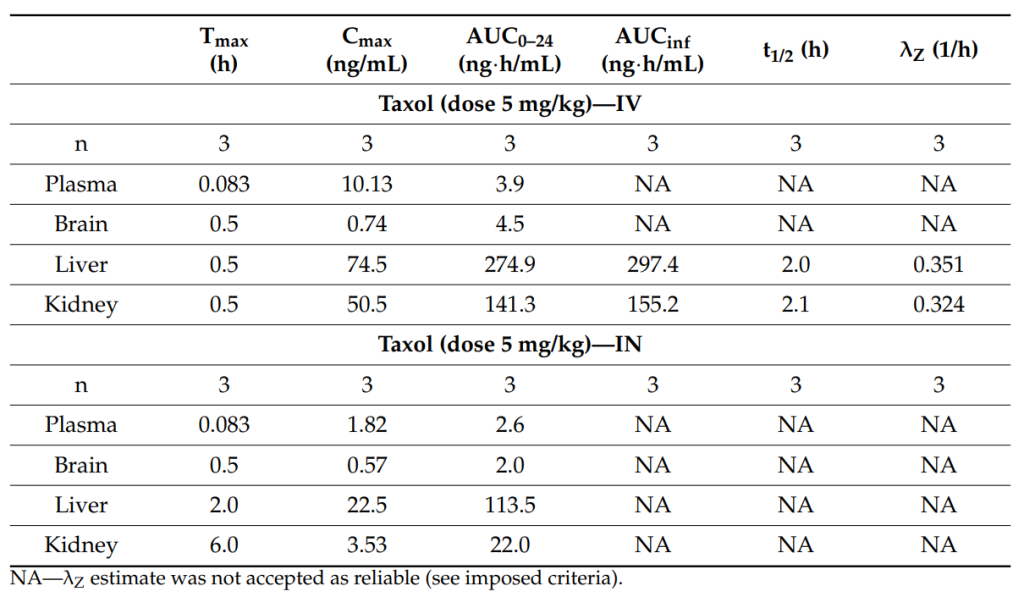
Table 1. Pharmacokinetic parameters of PTX following IN and IV administration to male SD rats.
Significance of the Result: The results demonstrate that both intravenous and intranasal administration of PTX-loaded PLGA nanoparticles result in appreciable drug accumulation in brain tissues, which is crucial for effective glioblastoma treatment. This indicates that the developed nanoparticles can cross the blood-brain barrier, an essential feature for treating brain tumors.
Key Innovations: The ability to achieve drug accumulation in the brain following intranasal administration is a novel aspect of this study. Intranasal delivery offers a non-invasive alternative to intravenous administration, which is typically associated with higher risk and complexity in clinical settings.
4. In Vitro Anti-Proliferative Effect on Glioblastoma Cells
Key Steps: The anti-proliferative effects of PTX-loaded PLGA nanoparticles were assessed using the U87MG glioblastoma cell line. Cells were treated with either blank PLGA nanoparticles or PTX-loaded PLGA nanoparticles at two different concentrations (10 µM and 25 µM) for 48 hours. The viability of the cells was measured using the MTT assay, which quantifies cell metabolic activity as a proxy for cell viability.
Result and Key Data: The study showed that, at the 10 µM concentration, there was no significant difference in cell viability between the PTX-loaded nanoparticles and free PTX. However, at 25 µM, the PTX-loaded nanoparticles significantly outperformed free PTX in reducing the viability of glioblastoma cells, indicating enhanced anti-cancer activity.
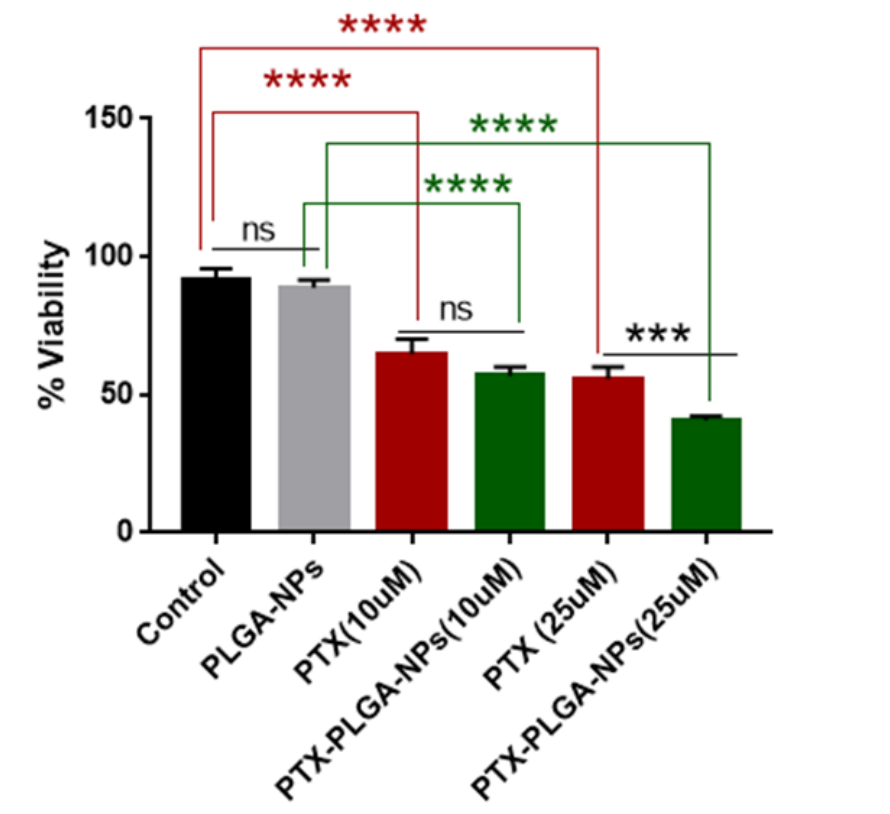
Figure 3. PTX-loaded nanoparticles are more effective than PTX alone in reducing the viability of GBM cells. Cell viability was determined by performing an MTT assay on U87MG cells 48 h after treatment with the indicated PTX concentrations. Statistical analyses of mean ± SD (standard deviation) were performed using Prism GraphPad 9.5.1.733. *** p < 0.0005, **** p < 0.0001, ns: not significant.
Significance of the Result: The enhanced anti-proliferative activity observed at higher concentrations of PTX-loaded nanoparticles suggests that the PLGA-based delivery system improves cellular uptake and drug release, offering a more effective treatment for glioblastoma. This result highlights the potential of PLGA nanoparticles to increase the efficacy of paclitaxel in treating resistant tumors like glioblastoma.
Key Innovations: The finding that PTX-loaded PLGA nanoparticles have superior anti-proliferative effects compared to free paclitaxel at higher concentrations demonstrates the potential of this nanoparticle system to enhance drug delivery and therapeutic outcomes in glioblastoma treatment.
5. Safety and Toxicity Evaluation
Key Steps: The safety of PTX-loaded PLGA nanoparticles was evaluated by monitoring the animals for any signs of toxicity, abnormal behavior, or organ damage. Rats were observed throughout the study, and their organ weights (brain, liver, kidney) were compared to control groups. Histopathological analysis of tissues was performed to assess any potential damage caused by the nanoparticles.
Result and Key Data: No significant toxicity or abnormal behavior was observed in any of the animals treated with PTX-loaded PLGA nanoparticles via either administration route. The organ weights of the treated animals were comparable to the control group, and no abnormal tissue appearance was detected.
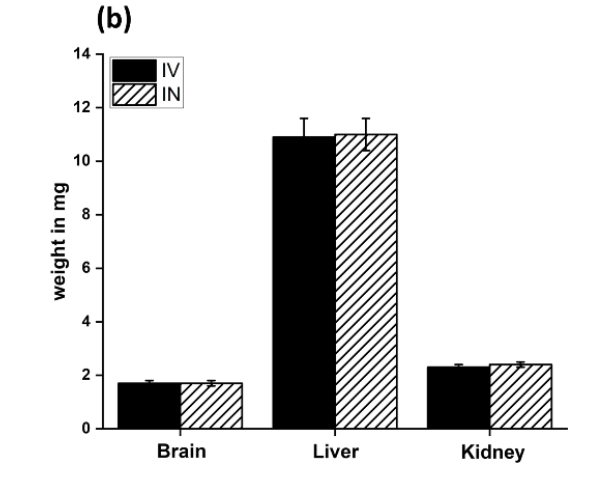
Figure 4. Weights of the various organs after the IN and IV administration of the PLGA NPs.
Significance of the Result: The lack of toxicity and organ damage associated with the PTX-loaded PLGA nanoparticles is a promising finding, as it indicates that this delivery system is safe for therapeutic use. This is crucial for clinical translation, where safety is a key consideration.
Key Innovations: The development of a paclitaxel delivery system that is both effective and non-toxic represents a significant innovation in the field of cancer nanomedicine. This dual benefit increases the potential for clinical use of PTX-loaded PLGA nanoparticles for glioblastoma treatment.
Conclusion
The study successfully demonstrates that paclitaxel-loaded PLGA nanoparticles can be delivered to the brain via both intravenous and intranasal routes, with significant accumulation in brain tissues and no observed toxicity. This research contributes to the field of drug delivery systems by offering a non-invasive method to treat glioblastoma more effectively. The findings suggest that this nanoparticle-based delivery system has potential applications in treating brain tumors, with the added benefit of reducing systemic side effects. However, the research is limited by the need for further optimization of the intranasal delivery method to improve drug concentration in the brain. Future studies should explore the long-term efficacy and safety of these nanoparticles, as well as their ability to deliver other chemotherapeutic agents to the brain.
Reference:
AbdEl-Haq, Muhammad, et al. “Paclitaxel delivery to the brain for glioblastoma treatment.” International Journal of Molecular Sciences 24.14 (2023): 11722.
