Editor: Sarah
A recent review underscores the growing importance of lipid nanoparticles (LNPs) in the delivery of biologics, such as therapeutic proteins, nucleic acids, and mRNA. As biologics play an increasingly critical role in clinical medicine, overcoming the challenges associated with their delivery has become a major focus, and LNPs have proven to be an effective solution. The review highlights LNPs as a promising delivery system due to their ability to enhance the stability, bioavailability, and targeted delivery of complex therapeutic agents.
Contributions and Key Findings
LNP Advantages for Biologics Delivery:
- LNPs provide a versatile and efficient delivery system due to their small size, biocompatibility, and biodegradability, making them ideal for transporting complex therapeutic agents without causing significant toxicity.
- These nanoparticles have shown particular promise in the delivery of biologics, such as therapeutic proteins, nucleic acids, and mRNA, which are often difficult to deliver effectively due to their size and sensitivity.

Figure 1: different types of LNPs with characteristics.
Influence of LNP Properties on Drug Delivery:
- The review emphasizes that the structure of LNPs, including lipid composition, surface charge, and particle size, directly affects drug encapsulation, release rates, and targeting capabilities.
- Different lipid types, such as cationic, ionizable, and PEGylated lipids, play a vital role in stabilizing LNPs, improving encapsulation efficiency, and protecting drugs from degradation.
Lipid Formulations and Manufacturing Strategies:
- Several lipid formulations and production strategies have been explored to enhance LNP performance. This includes the optimization of lipid compositions to create stable, effective LNPs capable of encapsulating large biologic molecules.
- Key lipid components such as ionizable lipids have been instrumental in improving the delivery of RNA-based therapeutics, including mRNA vaccines, by addressing challenges such as instability and poor cellular uptake.
RNA-Based Therapies:
- The success of LNPs in RNA-based therapies, including their use in COVID-19 vaccines, has been highlighted as a significant achievement. These systems demonstrated the ability to enhance the stability of mRNA and improve its cellular uptake, thus paving the way for future gene therapies and personalized medicine.
Technical Challenges in LNP Production:
- Despite their advantages, the production of LNPs at scale presents several challenges. High-pressure homogenization (HPH) is commonly used for large-scale LNP production, but issues such as lipid crystallization and drug degradation during processing need to be addressed.
- Emerging techniques like flash nanocomplexation and microfluidic methods show promise in improving the precision and scalability of LNP production, though further research is needed to optimize these methods for industrial applications.
Impact on Biopharmaceuticals and Personalized Medicine:
- The review emphasizes the broad implications of LNPs for the pharmaceutical industry, particularly in the context of RNA-based therapeutics, vaccines, and cancer immunotherapies. LNPs are being explored as a means to address challenges in the targeted delivery of biologic drugs.
- The ongoing development of more efficient and scalable LNP manufacturing processes could facilitate the delivery of treatments for genetic disorders, cancer, and other difficult-to-treat diseases.
Manufacturing Challenges and Solutions:
The review also delves into various techniques for manufacturing LNPs, which include high-energy, low-energy, and organic solvent-based methods. Each method has its advantages and limitations:
- High-Energy Methods: These methods, such as high-pressure homogenization (HPH), are widely used due to their scalability and efficiency. However, they are energy-intensive and can result in lipid crystallization and drug degradation during the process.
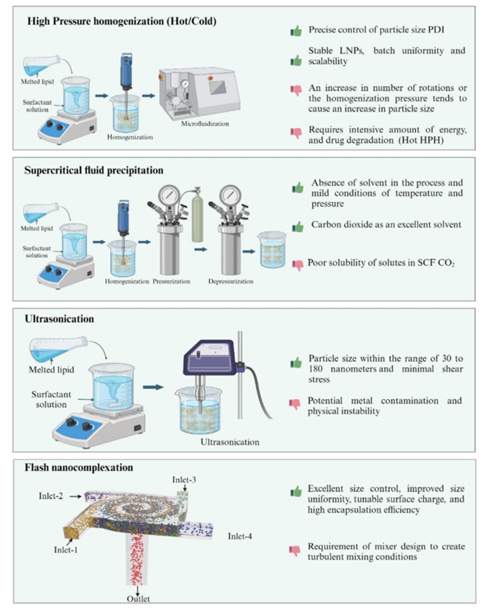
Figure 2: High-energy methods
- Low-Energy Methods: Techniques like thin-film hydration and reverse phase evaporation offer gentler approaches to LNP preparation, but they can be less effective in producing highly uniform particles.
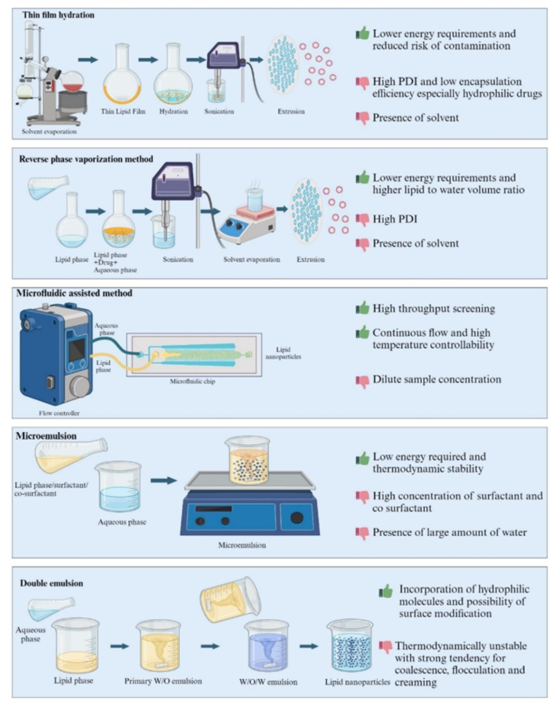
Figure 3: Low-energy methods
- Organic Solvent-Based Methods: These methods, including solvent emulsification and solvent injection, are typically used for small-scale production, but they may face challenges in removing organic solvents and ensuring the stability of the final product.
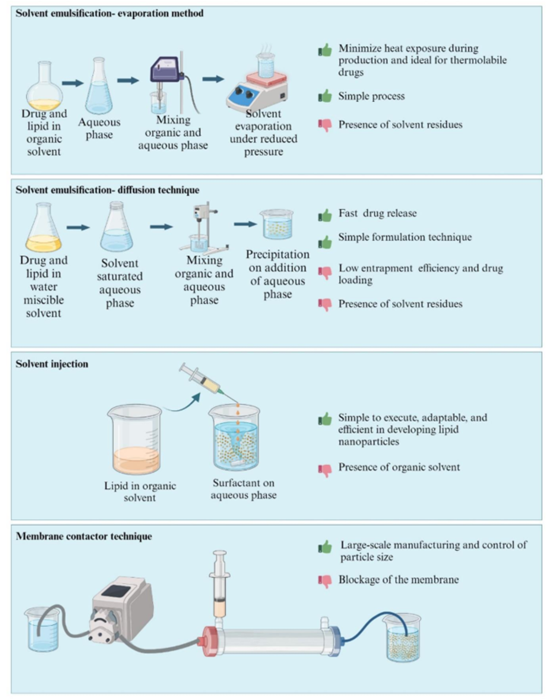
Figure 4: Organic Solvent-Based Methods
Implications for Biologics Delivery:
The findings of this study highlight the potential of LNP technology to address key challenges in biologics delivery, especially in the context of RNA-based drugs, vaccines, and gene therapies. As the need for improved biologic delivery systems grows, LNPs are emerging as a viable solution for delivering complex therapeutic agents. The future of LNP technology will depend on continued advancements in lipid formulations, regulatory approvals, and the optimization of manufacturing techniques.
Looking ahead, the review suggests that further research is needed to improve the scalability, reproducibility, and efficiency of LNP production. This could open new opportunities for the treatment of genetic disorders, cancers, and other conditions that currently lack effective treatments.
Reference
John, Rijo, et al. “Chemistry and Art of Developing Lipid Nanoparticles for Biologics Delivery: Focus on Development and Scale-Up.” Pharmaceutics, vol. 16, no. 1, 2024, p. 131. MDPI, https://doi.org/10.3390/pharmaceutics16010131.
